正在加载图片...
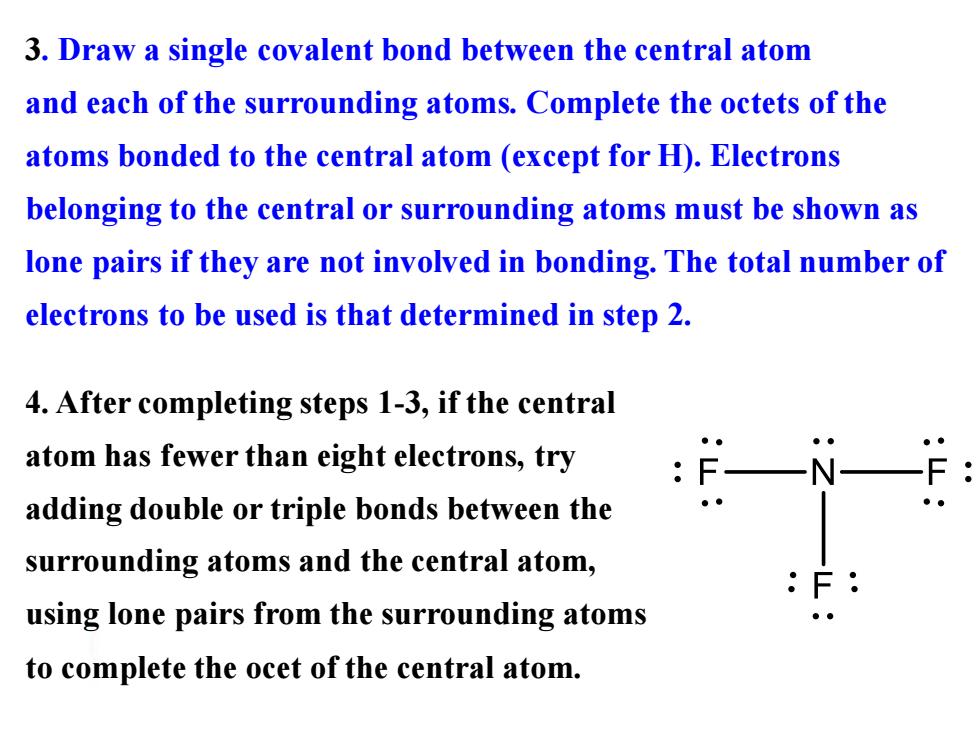
3.Draw a single covalent bond between the central atom and each of the surrounding atoms.Complete the octets of the atoms bonded to the central atom (except for H).Electrons belonging to the central or surrounding atoms must be shown as lone pairs if they are not involved in bonding.The total number of electrons to be used is that determined in step 2. 4.After completing steps 1-3,if the central atom has fewer than eight electrons,try adding double or triple bonds between the surrounding atoms and the central atom, using lone pairs from the surrounding atoms to complete the ocet of the central atom.3. Draw a single covalent bond between the central atom and each of the surrounding atoms. Complete the octets of the atoms bonded to the central atom (except for H). Electrons belonging to the central or surrounding atoms must be shown as lone pairs if they are not involved in bonding. The total number of electrons to be used is that determined in step 2. 4. After completing steps 1-3, if the central atom has fewer than eight electrons, try adding double or triple bonds between the surrounding atoms and the central atom, using lone pairs from the surrounding atoms to complete the ocet of the central atom