正在加载图片...
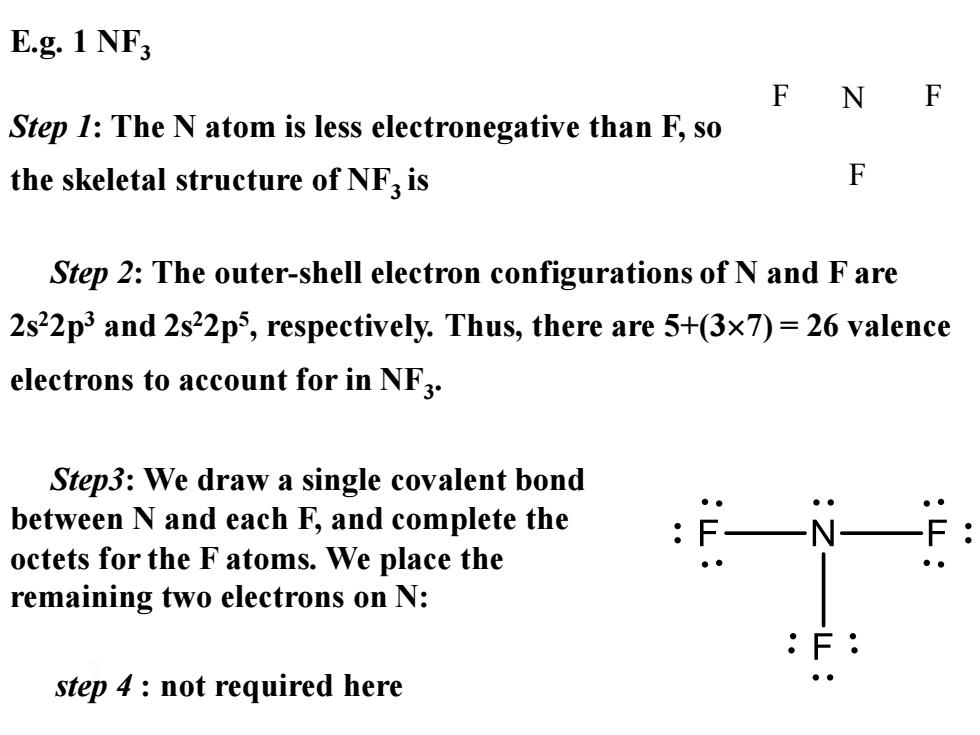
E.g.1 NF F F Step 1:The N atom is less electronegative than F,so the skeletal structure of NF3 is F Step 2:The outer-shell electron configurations of N and F are 2s22p3 and 2s22p5,respectively.Thus,there are 5+(3x7)=26 valence electrons to account for in NF3. Step3:We draw a single covalent bond between N and each F,and complete the octets for the F atoms.We place the remaining two electrons on N: step 4 not required here ● E.g. 1 NF3 Step 1: The N atom is less electronegative than F, so the skeletal structure of NF3 is F N F F Step3: We draw a single covalent bond between N and each F, and complete the octets for the F atoms. We place the remaining two electrons on N: Step 2: The outer-shell electron configurations of N and F are 2s22p3 and 2s22p5 , respectively. Thus, there are 5+(37) = 26 valence electrons to account for in NF3 . step 4 : not required here