正在加载图片...
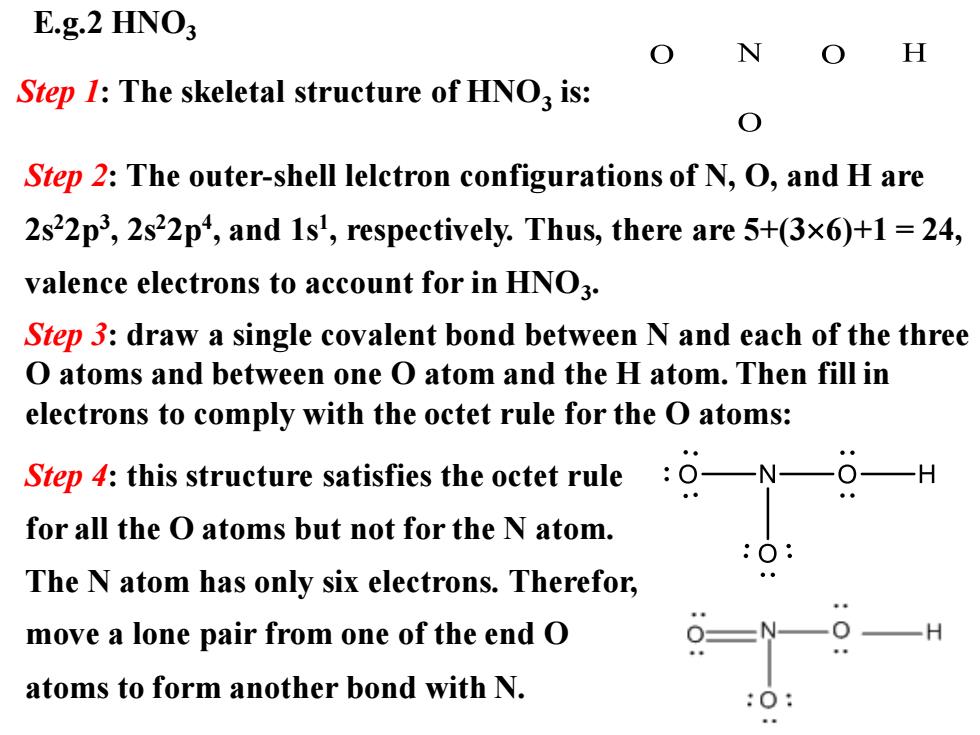
E.g.2 HNO3 N H Step 1:The skeletal structure of HNO3 is: Step 2:The outer-shell lelctron configurations of N,O,and H are 2s22p3,2s22p4,and 1s',respectively.Thus,there are 5+(3x6)+1=24, valence electrons to account for in HNO3. Step 3:draw a single covalent bond between N and each of the three O atoms and between one O atom and the H atom.Then fill in electrons to comply with the octet rule for the O atoms: Step 4:this structure satisfies the octet rule O-N- for all the O atoms but not for the N atom. The N atom has only six electrons.Therefor, move a lone pair from one of the end O atoms to form another bond with N.E.g.2 HNO3 Step 1: The skeletal structure of HNO3 is: Step 2: The outer-shell lelctron configurations of N, O, and H are 2s22p3 , 2s22p4 , and 1s1 , respectively. Thus, there are 5+(36)+1 = 24, valence electrons to account for in HNO3 . Step 4: this structure satisfies the octet rule for all the O atoms but not for the N atom. The N atom has only six electrons. Therefor, move a lone pair from one of the end O atoms to form another bond with N. Step 3: draw a single covalent bond between N and each of the three O atoms and between one O atom and the H atom. Then fill in electrons to comply with the octet rule for the O atoms: