正在加载图片...
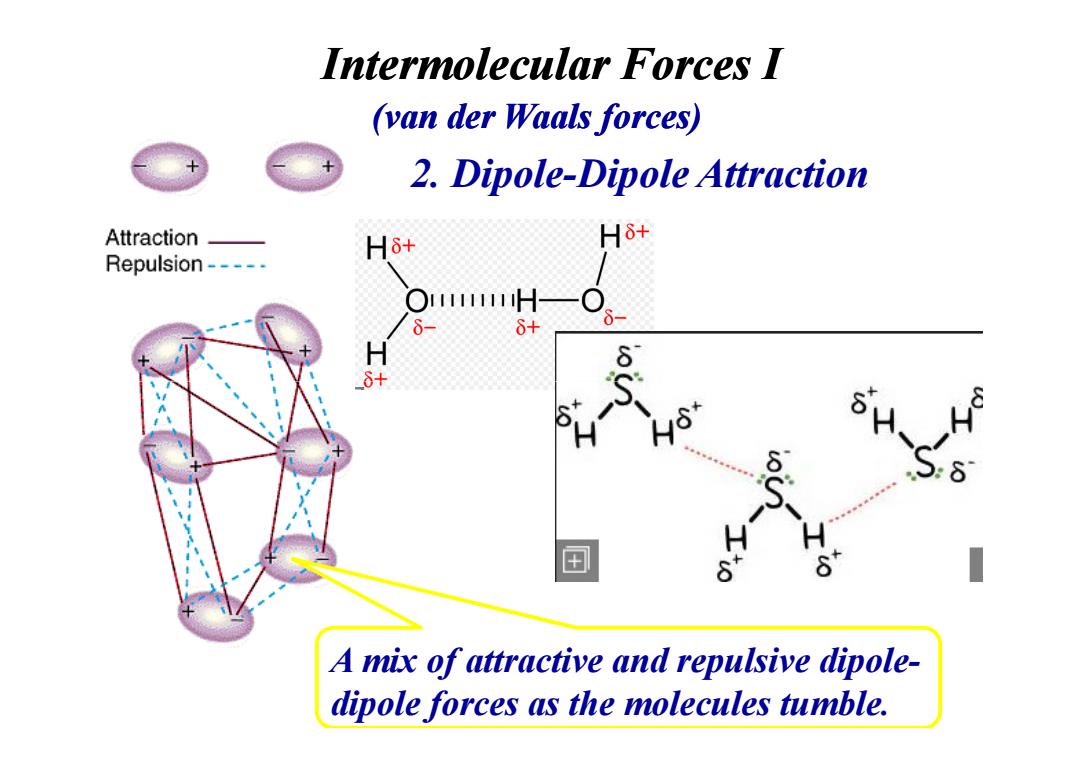
Intermolecular forces l (van der Waals forces) 2.Dipole-Dipole Attraction Attraction- Hδ+ Hδ+ Repulsion----- O11H 6- 6+ H A mix of attractive and repulsive dipole- dipole forces as the molecules tumble.2. Dipole-Dipole Attraction Intermolecular Forces I (van der Waals forces) A mix of attractive and repulsive dipoledipole forces as the molecules tumble