正在加载图片...
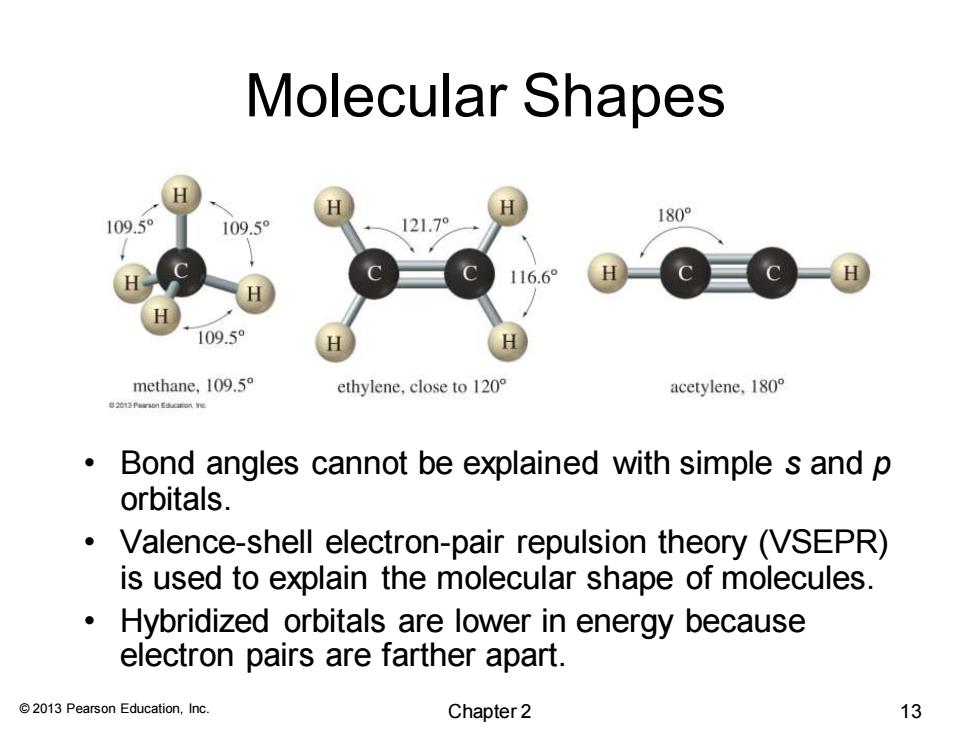
Molecular Shapes 180° 109.5° 109.5° 121.7° 116.6° 109.5 methane,109.5 ethylene,close to 120 acetylene,180 Bond angles cannot be explained with simple s and p orbitals. Valence-shell electron-pair repulsion theory (VSEPR) is used to explain the molecular shape of molecules. Hybridized orbitals are lower in energy because electron pairs are farther apart. 2013 Pearson Education,Inc. Chapter 2 13© 2013 Pearson Education, Inc. Molecular Shapes • Bond angles cannot be explained with simple s and p orbitals. • Valence-shell electron-pair repulsion theory (VSEPR) is used to explain the molecular shape of molecules. • Hybridized orbitals are lower in energy because electron pairs are farther apart. Chapter 2 13