正在加载图片...
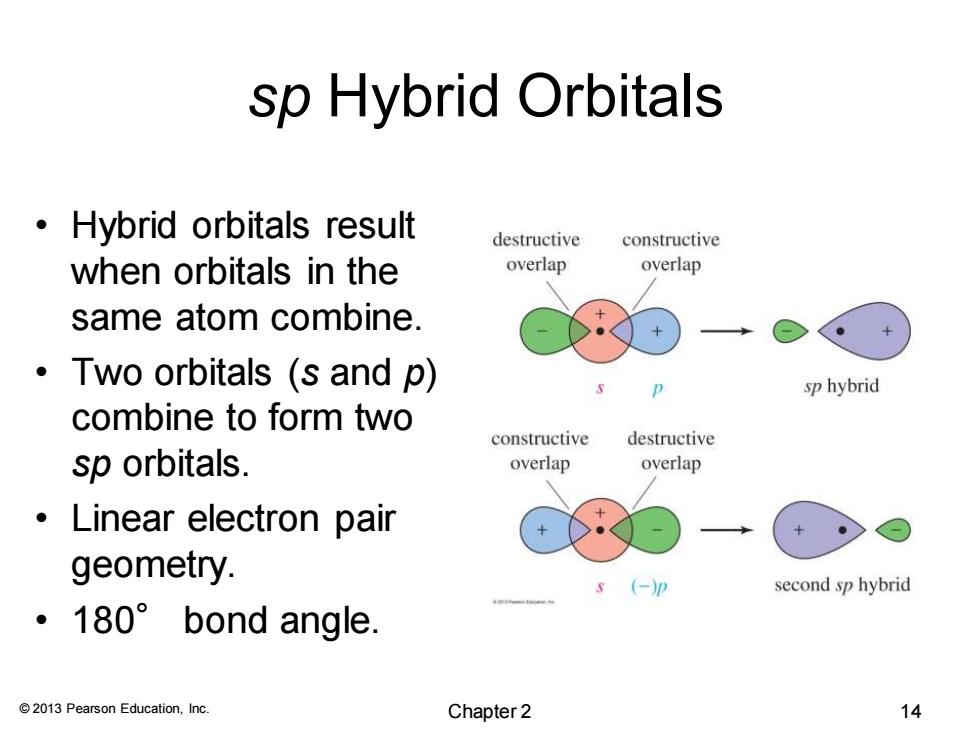
sp Hybrid Orbitals Hybrid orbitals result destructive constructive when orbitals in the overlap overlap same atom combine. ·Two orbitals(s and p) sp hybrid combine to form two constructive destructive sp orbitals. overlap overlap 。Linear electron pair geometry. s (-)p second sp hybrid ·180°bond angle. 2013 Pearson Education,Inc. Chapter 2 14 © 2013 Pearson Education, Inc. sp Hybrid Orbitals • Hybrid orbitals result when orbitals in the same atom combine. • Two orbitals (s and p) combine to form two sp orbitals. • Linear electron pair geometry. • 180° bond angle. Chapter 2 14