正在加载图片...
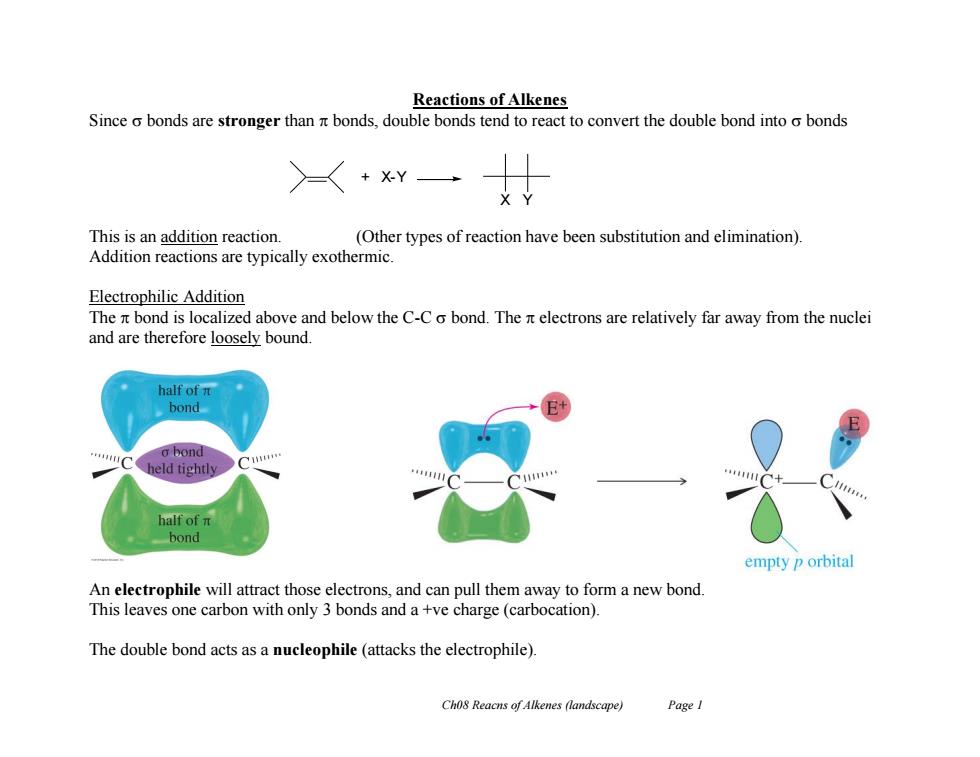
Reactions of Alkenes Since o bonds are stronger than nt bonds,double bonds tend to react to convert the double bond into o bonds >+xY This is an addition reaction (Other types of reaction have been substitution and elimination). Addition reactions are typically exothermic. Electrophilic Addition The it bond is localized above and below the C-C o bond.The it electrons are relatively far away from the nuclei and are therefore loosely bound. half of n bond o bond held tightly half of n bond empty p orbital An electrophile will attract those electrons,and can pull them away to form a new bond. This leaves one carbon with only 3 bonds and a +ve charge (carbocation). The double bond acts as a nucleophile(attacks the electrophile). Cho8 Reacns of Alkenes (landscape) Page ICh08 Reacns of Alkenes (landscape) Page 1 Reactions of Alkenes Since bonds are stronger than bonds, double bonds tend to react to convert the double bond into bonds This is an addition reaction. (Other types of reaction have been substitution and elimination). Addition reactions are typically exothermic. Electrophilic Addition The bond is localized above and below the C-C bond. The electrons are relatively far away from the nuclei and are therefore loosely bound. An electrophile will attract those electrons, and can pull them away to form a new bond. This leaves one carbon with only 3 bonds and a +ve charge (carbocation). The double bond acts as a nucleophile (attacks the electrophile). + X-Y X Y