正在加载图片...
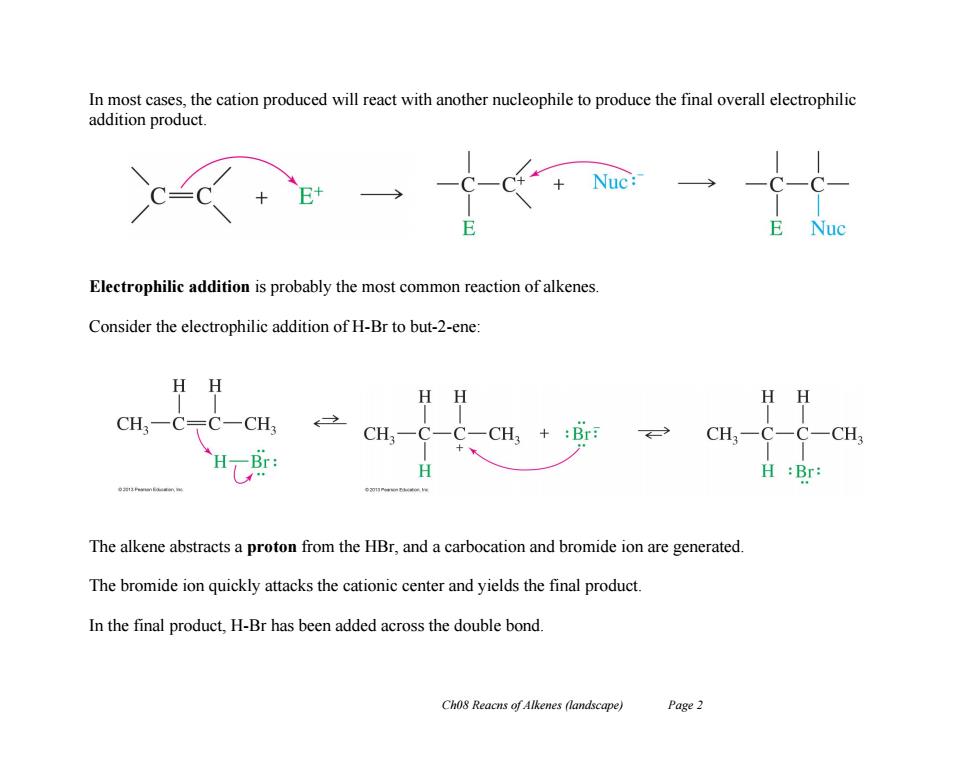
In most cases,the cation produced will react with another nucleophile to produce the final overall electrophilic addition product. Electrophilic addition is probably the most common reaction of alkenes. Consider the electrophilic addition of H-Br to but-2-ene: H HH CH. CH. CH, +:Br CH3一C-C-CH3 Br H:Br: The alkene abstracts a proton from the HBr,and a carbocation and bromide ion are generated. The bromide ion quickly attacks the cationic center and yields the final product. In the final product,H-Br has been added across the double bond. Cho8 Reacns of Alkenes (landscape) Page 2 Ch08 Reacns of Alkenes (landscape) Page 2 In most cases, the cation produced will react with another nucleophile to produce the final overall electrophilic addition product. Electrophilic addition is probably the most common reaction of alkenes. Consider the electrophilic addition of H-Br to but-2-ene: The alkene abstracts a proton from the HBr, and a carbocation and bromide ion are generated. The bromide ion quickly attacks the cationic center and yields the final product. In the final product, H-Br has been added across the double bond