正在加载图片...
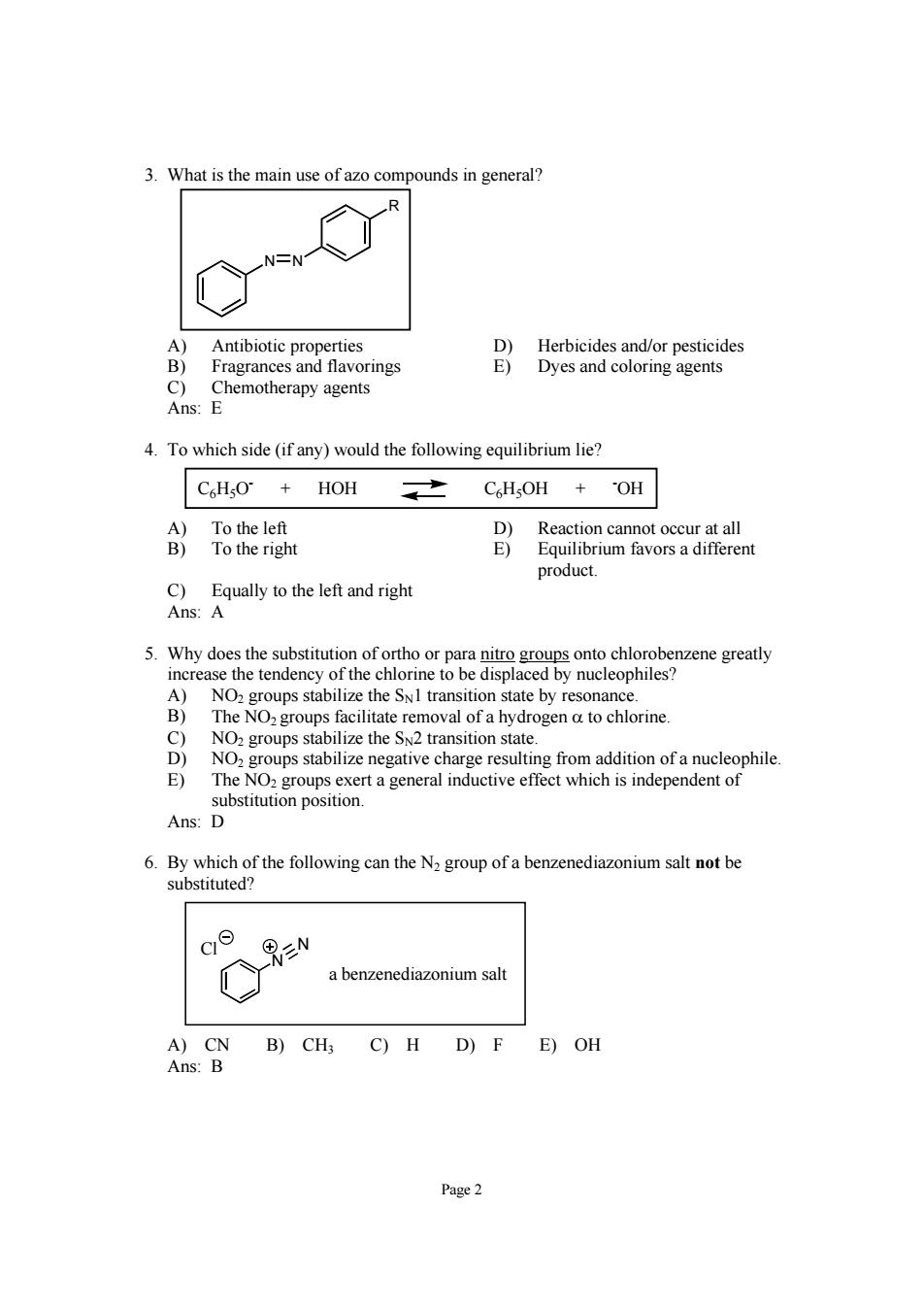
3.What is the main use of azo compounds in general? A) Antibiotic properties D)Herbicides and/or pesticides B) Fragrances and flavorings E)Dyes and coloring agents Chemotherapy agents Ans: 4.To which side(if any)would the following equilibrium lie? C6HO+HOH CHsOH +OH C)Equally to the left and right Ans:A 5.Why does the substitution of ortho or para nito groups onto chlorobenzene greatly the e to be displ d by nucleophiles n state by resc nanc sin a hy rogen a to chlorine NO. negative charge resulting from addition of a nucleophile E) The NOz groups exert a general inductive effect which is independent of substitution position. Ans:D 6.By which of the following can the N group of a benzenediazonium salt not be substituted? c N a benzenediazonium salt A)CN B)CH3 C)H D)F E)OH Ans:B Page2 Page 2 3. What is the main use of azo compounds in general? N N R A) Antibiotic properties D) Herbicides and/or pesticides B) Fragrances and flavorings E) Dyes and coloring agents C) Chemotherapy agents Ans: E 4. To which side (if any) would the following equilibrium lie? C6H5O- + HOH C6H5OH + - OH A) To the left D) Reaction cannot occur at all B) To the right E) Equilibrium favors a different product. C) Equally to the left and right Ans: A 5. Why does the substitution of ortho or para nitro groups onto chlorobenzene greatly increase the tendency of the chlorine to be displaced by nucleophiles? A) NO2 groups stabilize the SN1 transition state by resonance. B) The NO2 groups facilitate removal of a hydrogen α to chlorine. C) NO2 groups stabilize the SN2 transition state. D) NO2 groups stabilize negative charge resulting from addition of a nucleophile. E) The NO2 groups exert a general inductive effect which is independent of substitution position. Ans: D 6. By which of the following can the N2 group of a benzenediazonium salt not be substituted? N N a benzenediazonium salt Cl A) CN B) CH3 C) H D) F E) OH Ans: B