正在加载图片...
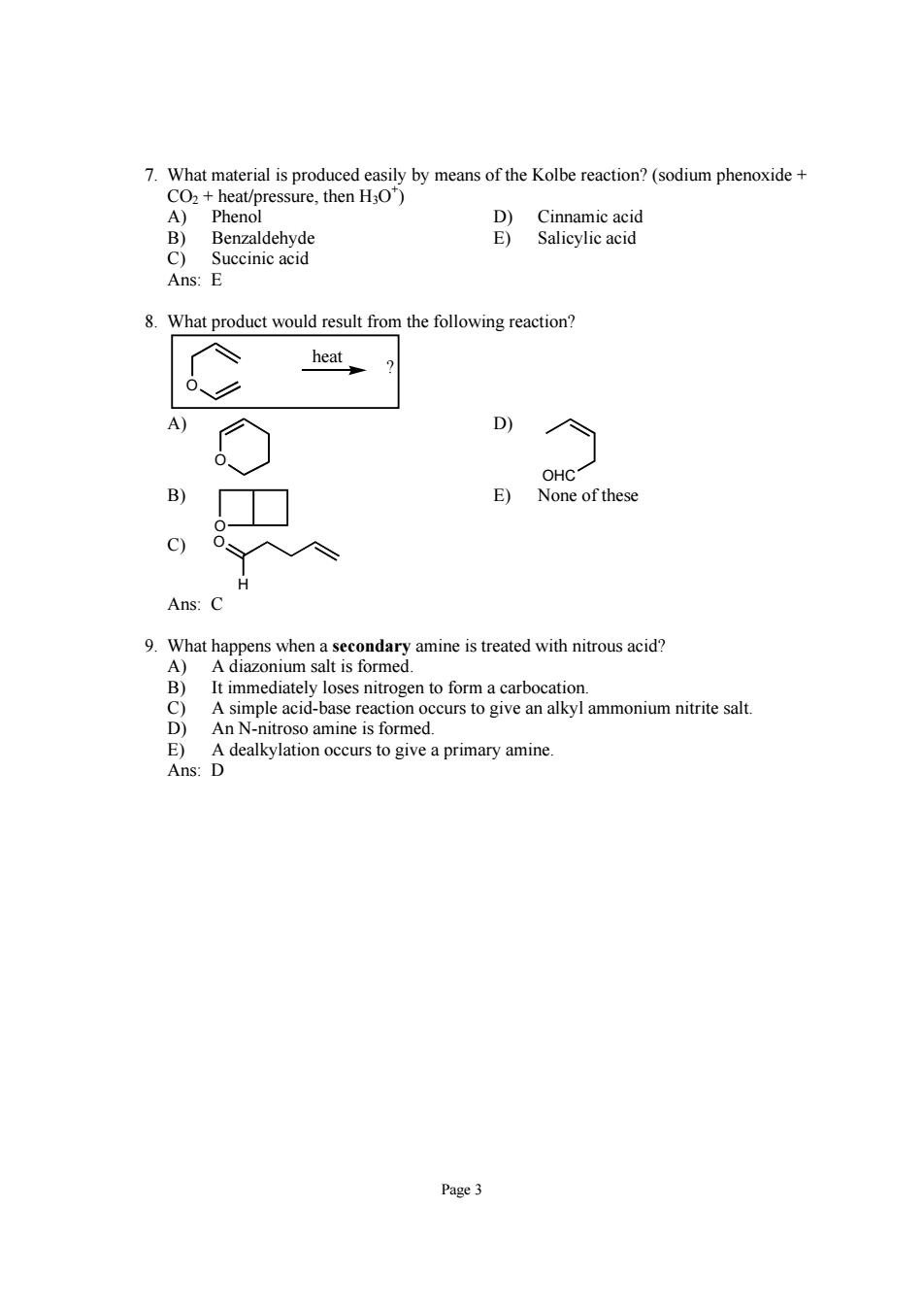
7.What material is produced easily by means of the Kolbe reaction?(sodium phenoxide heat/pressure,then HO) Succinic acid Ans:E 8.What product would result from the following reaction? heat D OHC E) None ofthese Ans: 9.What happens when a secondary amine is treated with nitrous acid? A)A diazonium salt is formed. It immediately loses nitrogen to form a carbocation. A simple acid-base reaction occurs to give an alkyl ammonium nitrite salt. troso amine is formed D dealkylation occurs to give a primary amine Page 3Page 3 7. What material is produced easily by means of the Kolbe reaction? (sodium phenoxide + CO2 + heat/pressure, then H3O+ ) A) Phenol D) Cinnamic acid B) Benzaldehyde E) Salicylic acid C) Succinic acid Ans: E 8. What product would result from the following reaction? O ? heat A) O D) OHC B) O E) None of these C) O H Ans: C 9. What happens when a secondary amine is treated with nitrous acid? A) A diazonium salt is formed. B) It immediately loses nitrogen to form a carbocation. C) A simple acid-base reaction occurs to give an alkyl ammonium nitrite salt. D) An N-nitroso amine is formed. E) A dealkylation occurs to give a primary amine. Ans: D