正在加载图片...
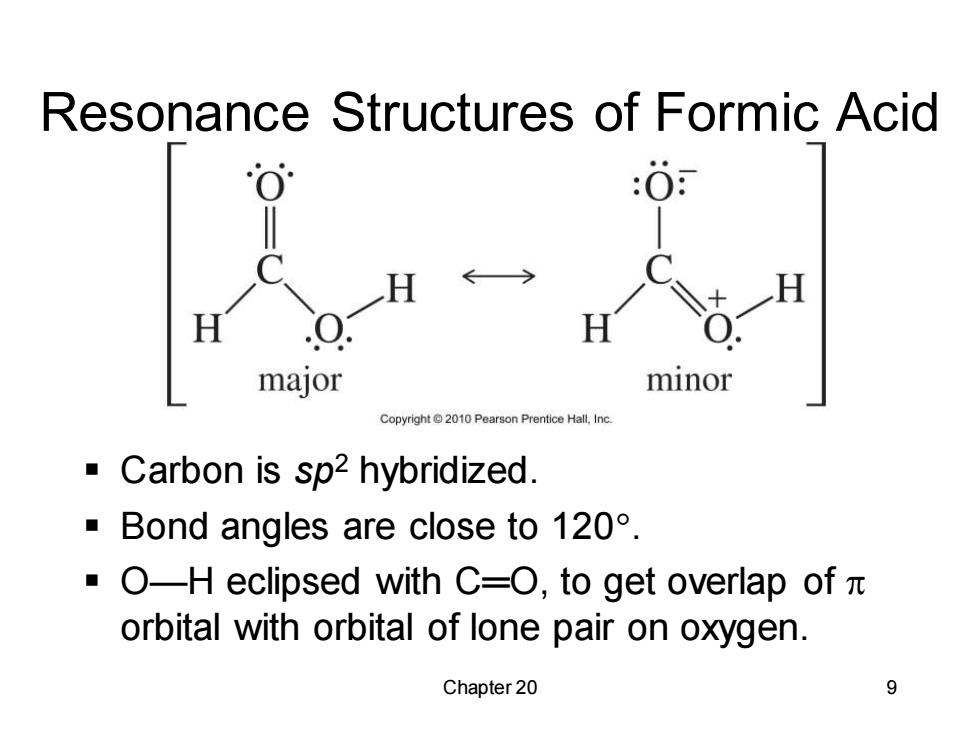
Resonance Structures of Formic Acid :0 H H major minor Copyright2010 Pearson Prentice Hall,Inc Carbon is sp2 hybridized. Bond angles are close to 120. ·O-H eclipsed with C=O,to get overlap ofπ orbital with orbital of lone pair on oxygen. Chapter 20 9Chapter 20 9 Resonance Structures of Formic Acid ▪ Carbon is sp2 hybridized. ▪ Bond angles are close to 120. ▪ O—H eclipsed with C═O, to get overlap of orbital with orbital of lone pair on oxygen