正在加载图片...
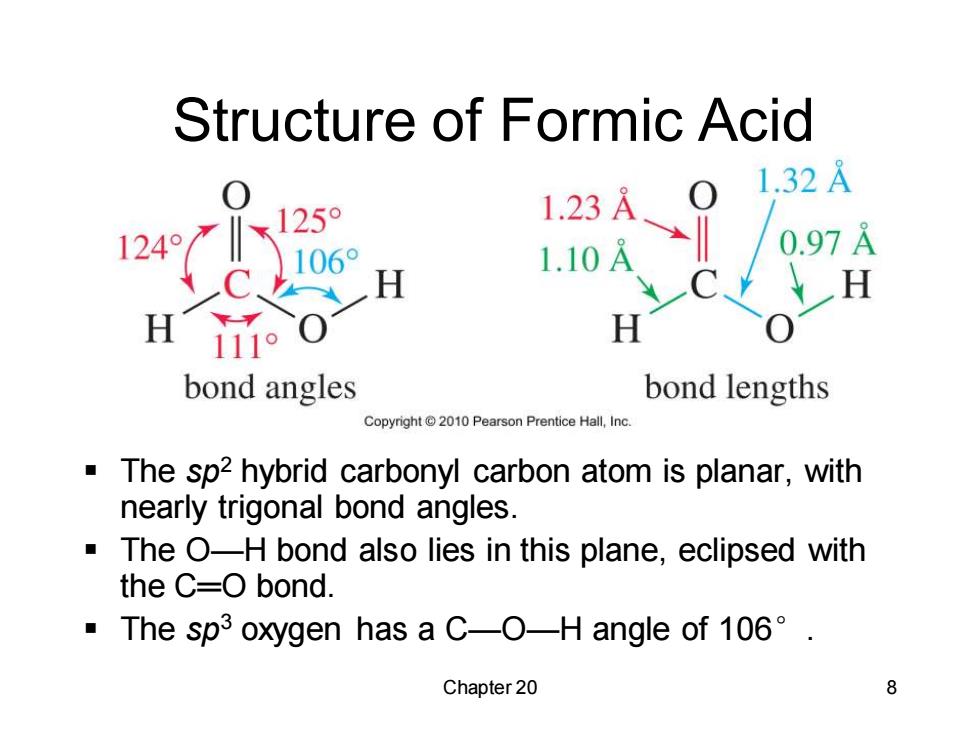
Structure of Formic Acid 1.32A 25° 1.23A 124° 1.10A 0.97A H H H 111o H bond angles bond lengths Copyright 2010 Pearson Prentice Hall,Inc The sp2 hybrid carbonyl carbon atom is planar,with nearly trigonal bond angles. The O-H bond also lies in this plane,eclipsed with the C=O bond. ·The spi3 oxygen has a C--O-H angle of106°. Chapter 20 8 Chapter 20 8 Structure of Formic Acid ▪ The sp2 hybrid carbonyl carbon atom is planar, with nearly trigonal bond angles. ▪ The O—H bond also lies in this plane, eclipsed with the C═O bond. ▪ The sp3 oxygen has a C—O—H angle of 106°