正在加载图片...
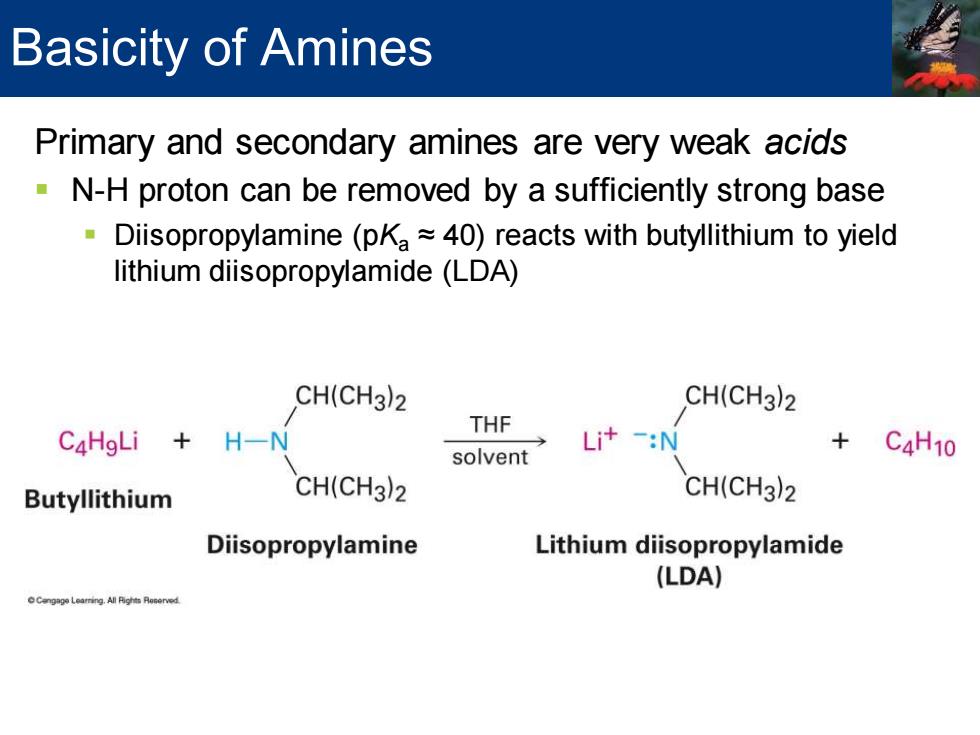
Basicity of Amines Primary and secondary amines are very weak acids N-H proton can be removed by a sufficiently strong base Diisopropylamine (pKa~40)reacts with butyllithium to yield lithium diisopropylamide(LDA) CH(CH3)2 CH(CH3)2 THF C4HgLi H-N Li+-:N solvent C4H10 Butyllithium CH(CH3)2 CH(CH3)2 Diisopropylamine Lithium diisopropylamide (LDA)Primary and secondary amines are very weak acids ▪ N-H proton can be removed by a sufficiently strong base ▪ Diisopropylamine (pKa ≈ 40) reacts with butyllithium to yield lithium diisopropylamide (LDA) Basicity of Amines