正在加载图片...
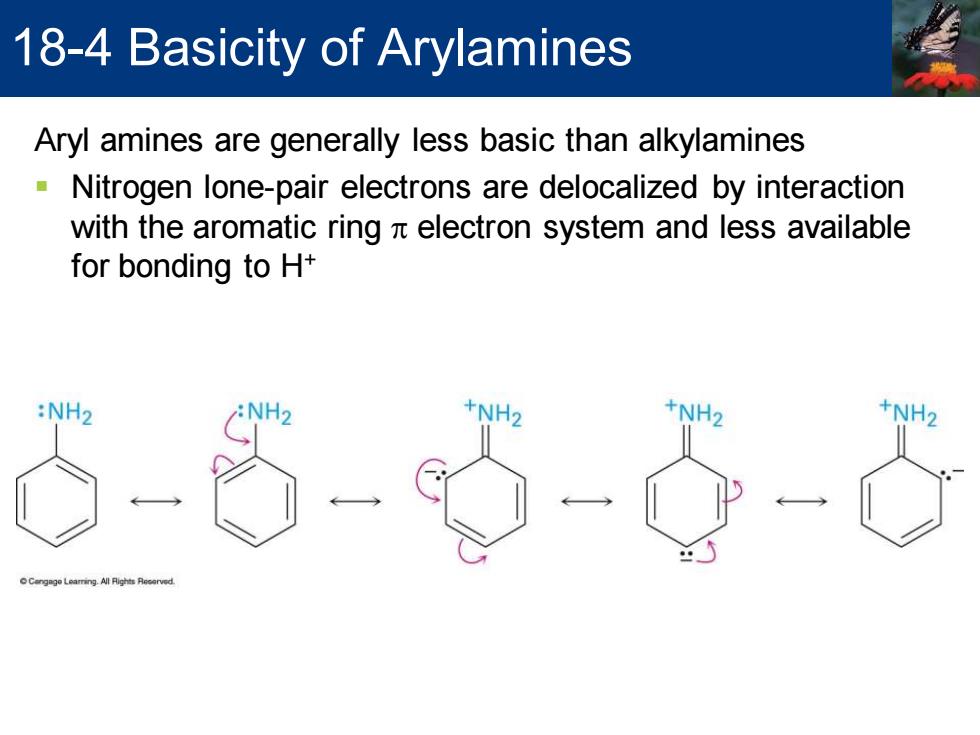
18-4 Basicity of Arylamines Aryl amines are generally less basic than alkylamines Nitrogen lone-pair electrons are delocalized by interaction with the aromatic ring x electron system and less available for bonding to H+ :NH2 +NH2 +NH2 Aryl amines are generally less basic than alkylamines ▪ Nitrogen lone-pair electrons are delocalized by interaction with the aromatic ring p electron system and less available for bonding to H+ 18-4 Basicity of Arylamines