正在加载图片...
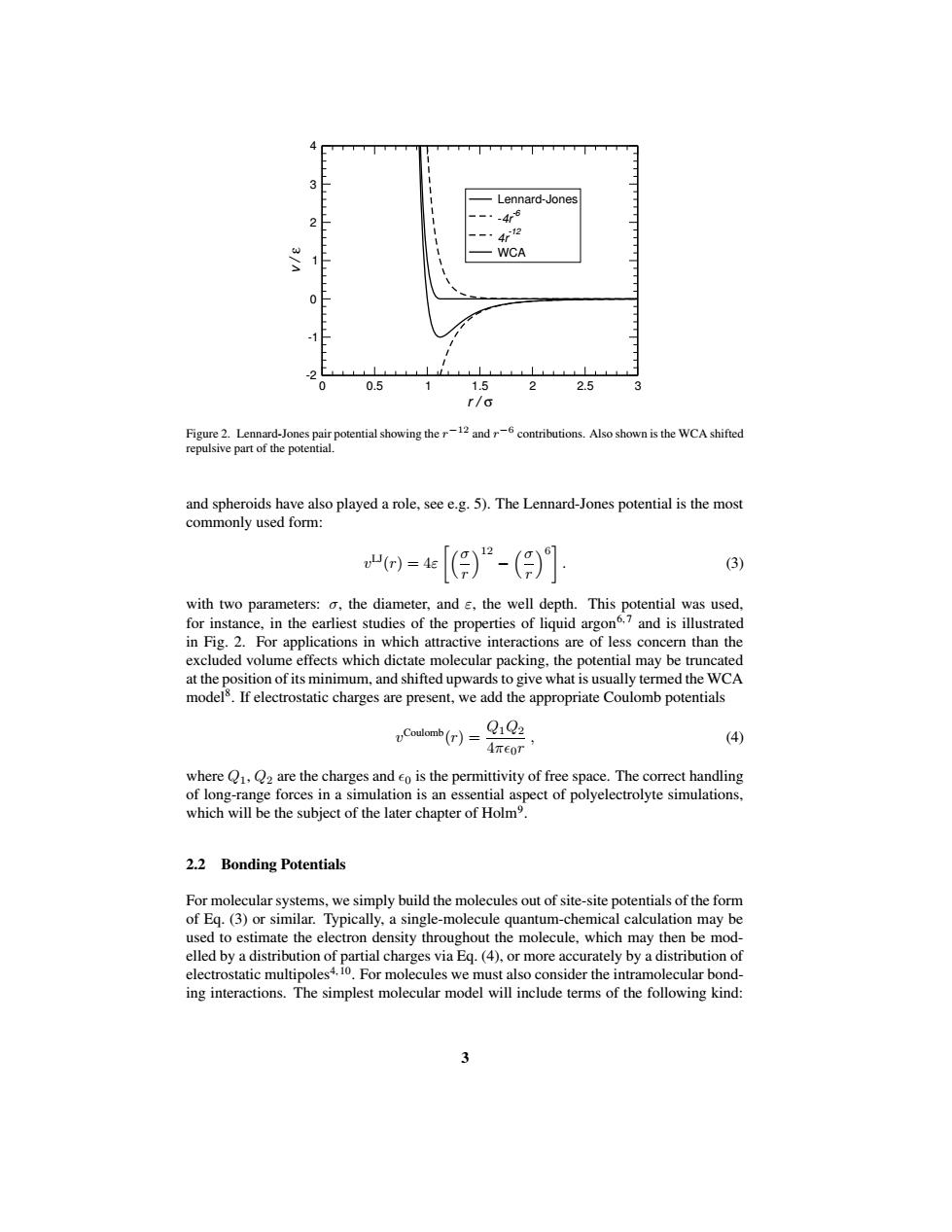
Lennard-Jones 4斯6 WCA 0 -1 0 0.5 1.5 2 2.5 3 r/6 Figure 2.Lennard-Jones pair potential showing.Also shown is the WCA shifted repulsive part of the potential. and spheroids have also played a role,see e.g.5).The Lennard-Jones potential is the most commonly used form: )=[))“-() (3) with two parameters:o,the diameter,and s,the well depth.This potential was used, for instance,in the earliest studies of the properties of liquid argon.7 and is illustrated in Fig.2.For applications in which attractive interactions are of less concern than the excluded volume effects which dictate molecular packing,the potential may be truncated at the position of its minimum,and shifted upwards to give what is usually termed the WCA models.If electrostatic charges are present,we add the appropriate Coulomb potentials Coulomb(r)= QiQ2 (4) 4Teor where Q1,Q2 are the charges and eo is the permittivity of free space.The correct handling of long-range forces in a simulation is an essential aspect of polyelectrolyte simulations, which will be the subject of the later chapter of Holm. 2.2 Bonding Potentials For molecular systems,we simply build the molecules out of site-site potentials of the form of Eg.(3)or similar.Typically,a single-molecule quantum-chemical calculation may be used to estimate the electron density throughout the molecule,which may then be mod- elled by a distribution of partial charges via Eq.(4),or more accurately by a distribution of electrostatic multipoles4.10.For molecules we must also consider the intramolecular bond- ing interactions.The simplest molecular model will include terms of the following kind:0 0.5 1 1.5 2 2.5 3 r / σ -2 -1 0 1 2 3 4 v / ε Lennard-Jones -4r-6 4r-12 WCA Figure 2. Lennard-Jones pair potential showing the r−12 and r−6 contributions. Also shown is the WCA shifted repulsive part of the potential. and spheroids have also played a role, see e.g. 5). The Lennard-Jones potential is the most commonly used form: v LJ(r) = 4ε σ r 12 − σ r 6 . (3) with two parameters: σ, the diameter, and ε, the well depth. This potential was used, for instance, in the earliest studies of the properties of liquid argon6, 7 and is illustrated in Fig. 2. For applications in which attractive interactions are of less concern than the excluded volume effects which dictate molecular packing, the potential may be truncated at the position of its minimum, and shifted upwardsto give what is usually termed the WCA model8 . If electrostatic charges are present, we add the appropriate Coulomb potentials v Coulomb(r) = Q1Q2 4π0r , (4) where Q1, Q2 are the charges and 0 is the permittivity of free space. The correct handling of long-range forces in a simulation is an essential aspect of polyelectrolyte simulations, which will be the subject of the later chapter of Holm9 . 2.2 Bonding Potentials For molecular systems, we simply build the molecules out of site-site potentials of the form of Eq. (3) or similar. Typically, a single-molecule quantum-chemical calculation may be used to estimate the electron density throughout the molecule, which may then be modelled by a distribution of partial charges via Eq. (4), or more accurately by a distribution of electrostatic multipoles4, 10 . For molecules we must also consider the intramolecular bonding interactions. The simplest molecular model will include terms of the following kind: 3