正在加载图片...
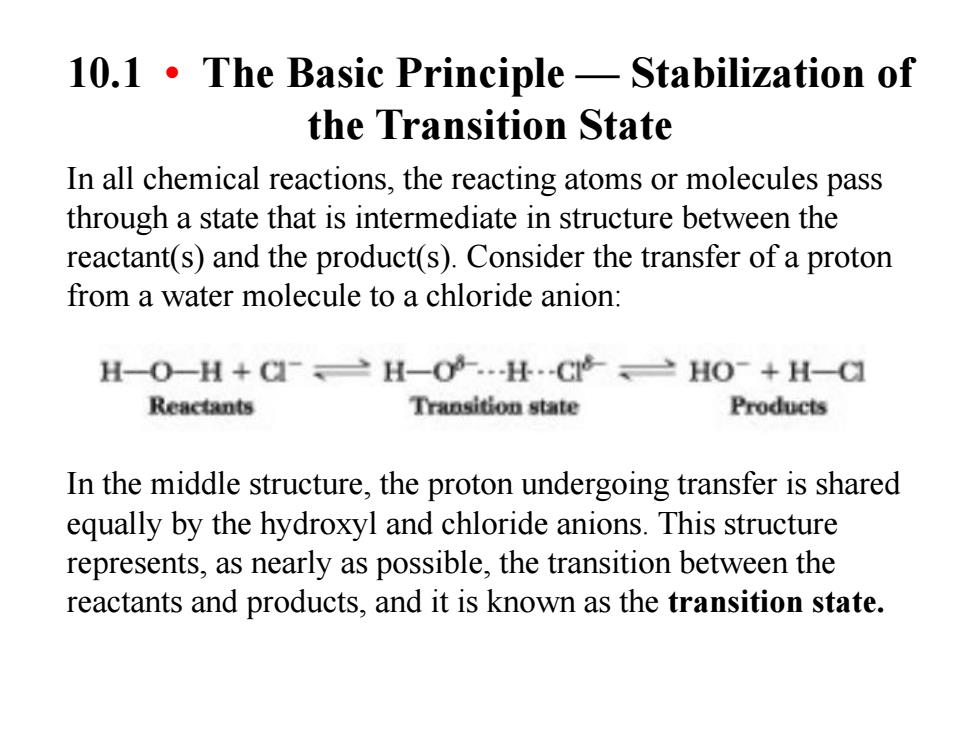
10.1.The Basic Principle-Stabilization of the Transition State In all chemical reactions,the reacting atoms or molecules pass through a state that is intermediate in structure between the reactant(s)and the product(s).Consider the transfer of a proton from a water molecule to a chloride anion: H-O-H+CH-H.C>HO-+H-C Reactants Transition state Products In the middle structure,the proton undergoing transfer is shared equally by the hydroxyl and chloride anions.This structure represents,as nearly as possible,the transition between the reactants and products,and it is known as the transition state. 10.1 • The Basic Principle — Stabilization of the Transition State In all chemical reactions, the reacting atoms or molecules pass through a state that is intermediate in structure between the reactant(s) and the product(s). Consider the transfer of a proton from a water molecule to a chloride anion: In the middle structure, the proton undergoing transfer is shared equally by the hydroxyl and chloride anions. This structure represents, as nearly as possible, the transition between the reactants and products, and it is known as the transition state