正在加载图片...
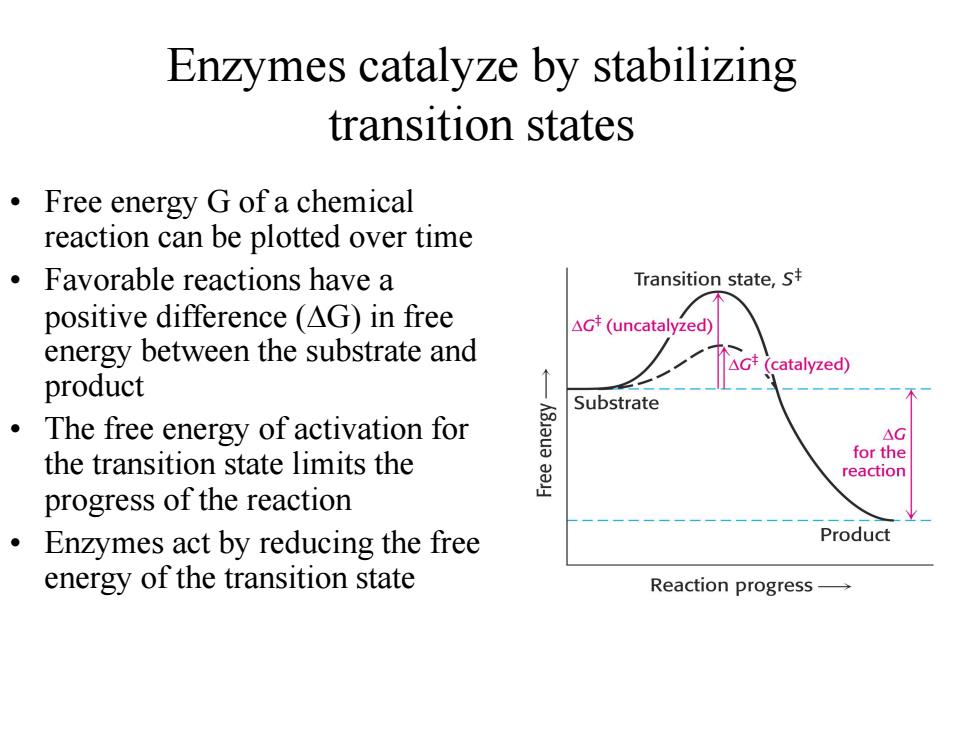
Enzymes catalyze by stabilizing transition states Free energy G of a chemical reaction can be plotted over time Favorable reactions have a Transition state,s positive difference (AG)in free △Gt(uncatalyzed energy between the substrate and catalyzed) product ò Substrate The free energy of activation for AG the transition state limits the for the reaction progress of the reaction Enzymes act by reducing the free Product energy of the transition state Reaction progressEnzymes catalyze by stabilizing transition states • Free energy G of a chemical reaction can be plotted over time • Favorable reactions have a positive difference (DG) in free energy between the substrate and product • The free energy of activation for the transition state limits the progress of the reaction • Enzymes act by reducing the free energy of the transition state