
Chapter 10 Mechanism of Enzyme Action Although the catalytic properties of enzymes may seem almost magical,it is simply chemistry-the breaking and making of bonds-that gives enzymes their prowess. This chapter will explore the unique features of this chemistry
Chapter 10 Mechanism of Enzyme Action Although the catalytic properties of enzymes may seem almost magical, it is simply chemistry—the breaking and making of bonds—that gives enzymes their prowess. This chapter will explore the unique features of this chemistry
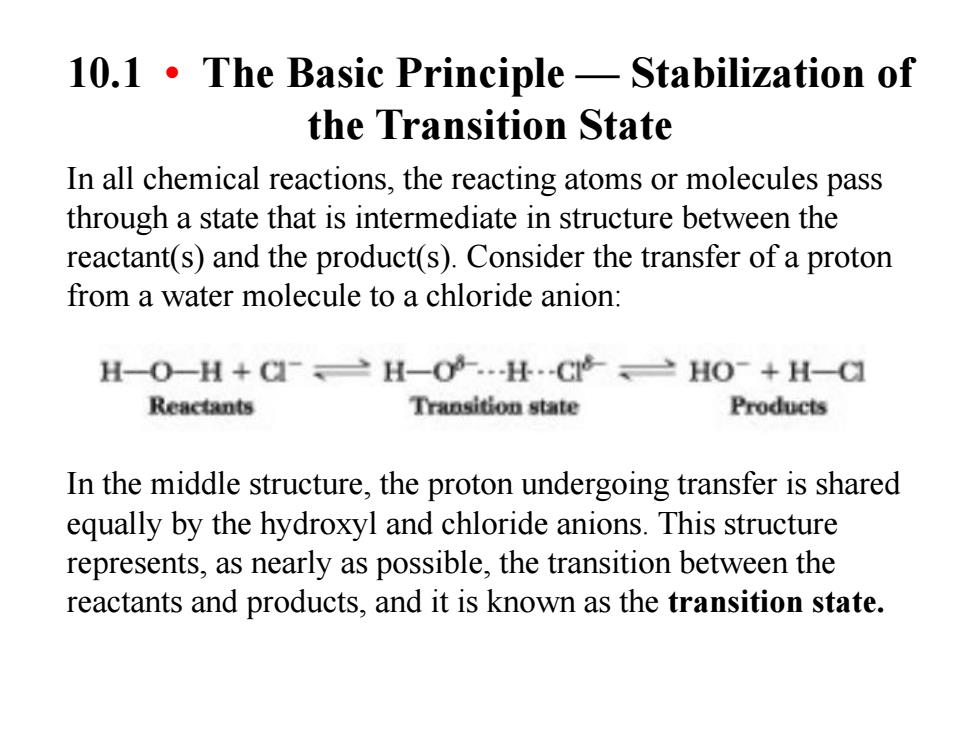
10.1.The Basic Principle-Stabilization of the Transition State In all chemical reactions,the reacting atoms or molecules pass through a state that is intermediate in structure between the reactant(s)and the product(s).Consider the transfer of a proton from a water molecule to a chloride anion: H-O-H+CH-H.C>HO-+H-C Reactants Transition state Products In the middle structure,the proton undergoing transfer is shared equally by the hydroxyl and chloride anions.This structure represents,as nearly as possible,the transition between the reactants and products,and it is known as the transition state
10.1 • The Basic Principle — Stabilization of the Transition State In all chemical reactions, the reacting atoms or molecules pass through a state that is intermediate in structure between the reactant(s) and the product(s). Consider the transfer of a proton from a water molecule to a chloride anion: In the middle structure, the proton undergoing transfer is shared equally by the hydroxyl and chloride anions. This structure represents, as nearly as possible, the transition between the reactants and products, and it is known as the transition state
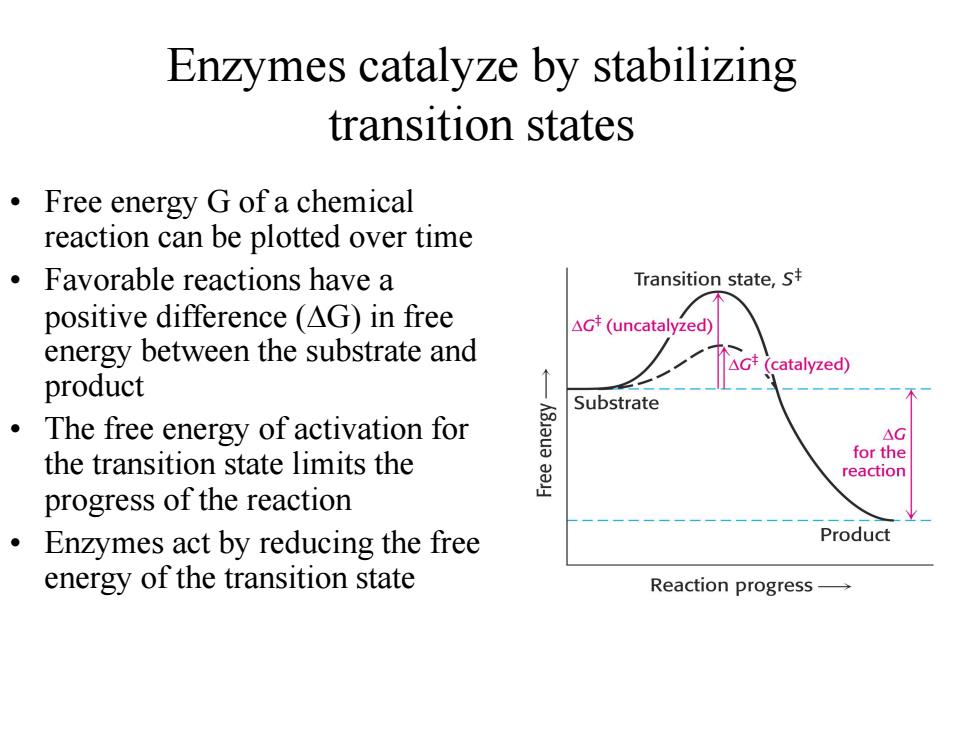
Enzymes catalyze by stabilizing transition states Free energy G of a chemical reaction can be plotted over time Favorable reactions have a Transition state,s positive difference (AG)in free △Gt(uncatalyzed energy between the substrate and catalyzed) product ò Substrate The free energy of activation for AG the transition state limits the for the reaction progress of the reaction Enzymes act by reducing the free Product energy of the transition state Reaction progress
Enzymes catalyze by stabilizing transition states • Free energy G of a chemical reaction can be plotted over time • Favorable reactions have a positive difference (DG) in free energy between the substrate and product • The free energy of activation for the transition state limits the progress of the reaction • Enzymes act by reducing the free energy of the transition state
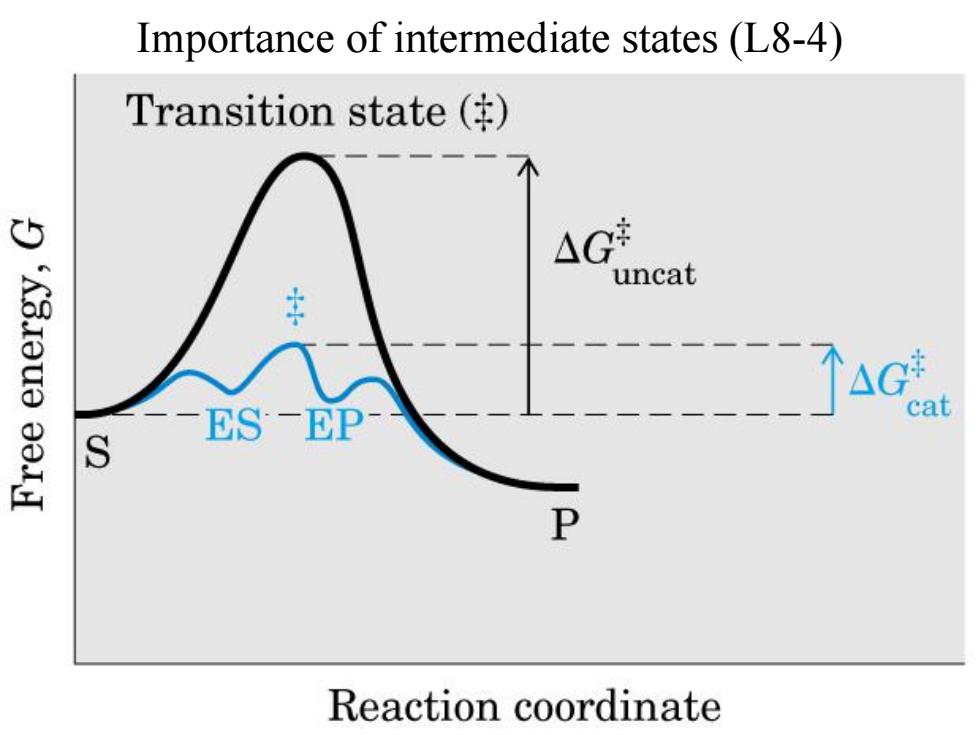
Importance of intermediate states (L8-4) Transition state ( 4 uncat -AGt -ES一EP at S P Reaction coordinate
Importance of intermediate states (L8-4)
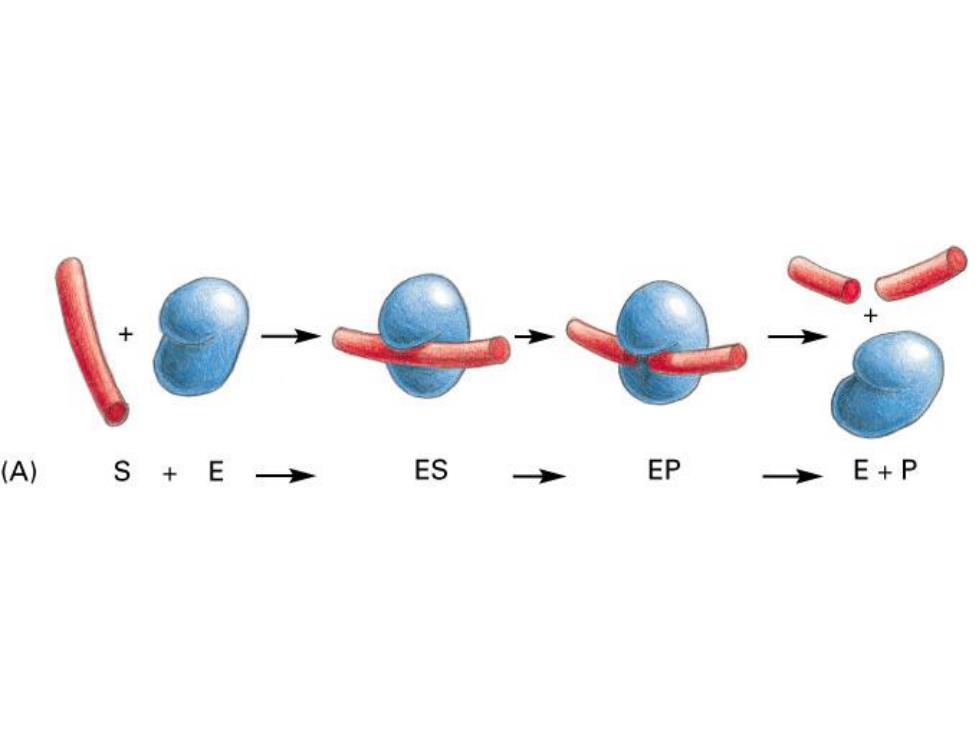
(A) S+E ES EP E+P

(A)HYDROLYSIS OF AN AMIDE BOND ● -N-H+ H tetrahedral water intermediate (B)TRANSITION-STATE ANALOG FOR AMIDE HYDROLYSIS HN HN NH NH O NO2 OH O analog amide Figure 3-47.Molecular Biology of the Cell,4th Edition

OH 6-0H R 0 R 0 →Products 0 08 Ester hydrolysis Transition state 06 R 0 R Analog (phosphonate) OH 8-0H H H-N H一】 →Products H NO NO Carbonate hydrolysis Transition state H 06 H-N H NO Box 8-3 Lehn Analog (phosphate)
Box 8-3 Lehn
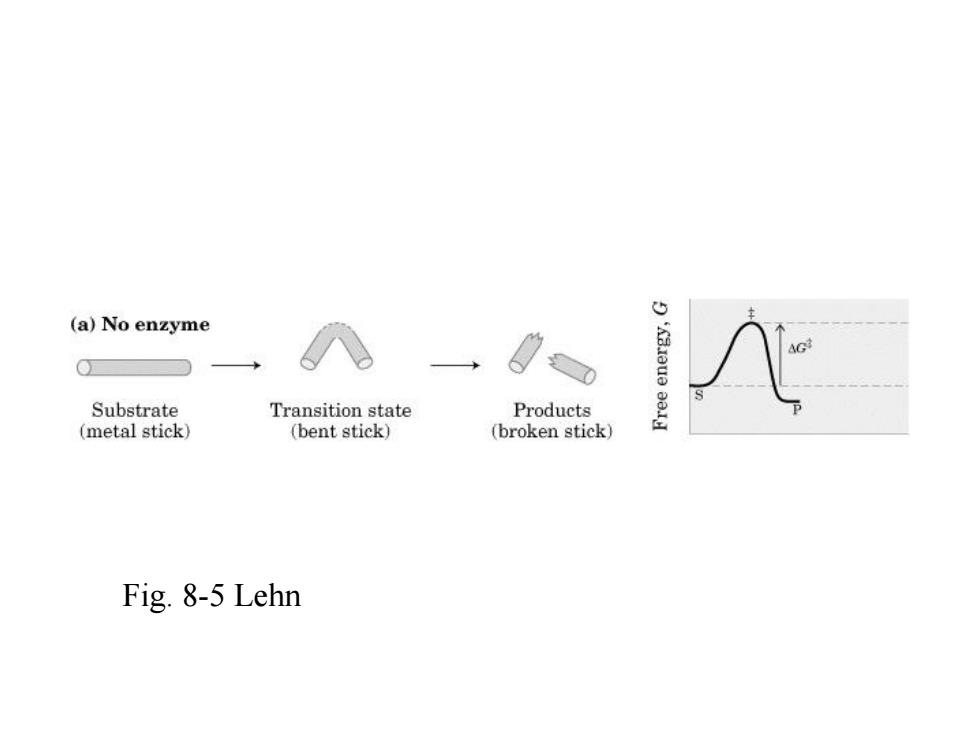
(a)No enzyme Substrate Transition state Products (metal stick) (bent stick) (broken stick) Fig.8-5 Lehn
Fig. 8-5 Lehn
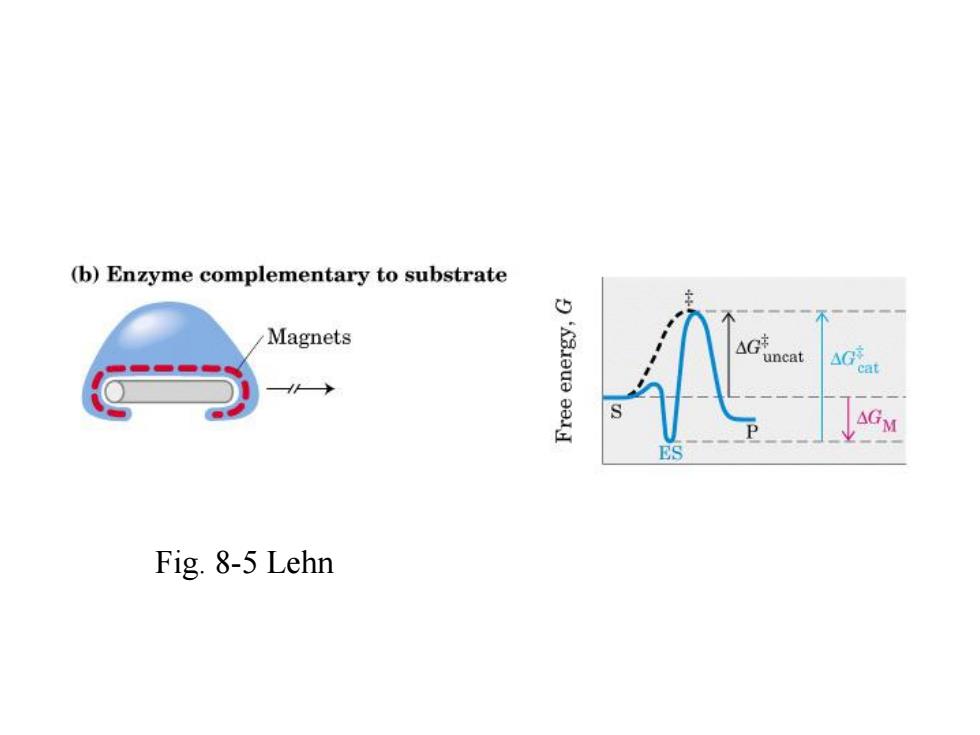
(b)Enzyme complementary to substrate Magnets Gancat → ES Fig.8-5 Lehn
Fig. 8-5 Lehn
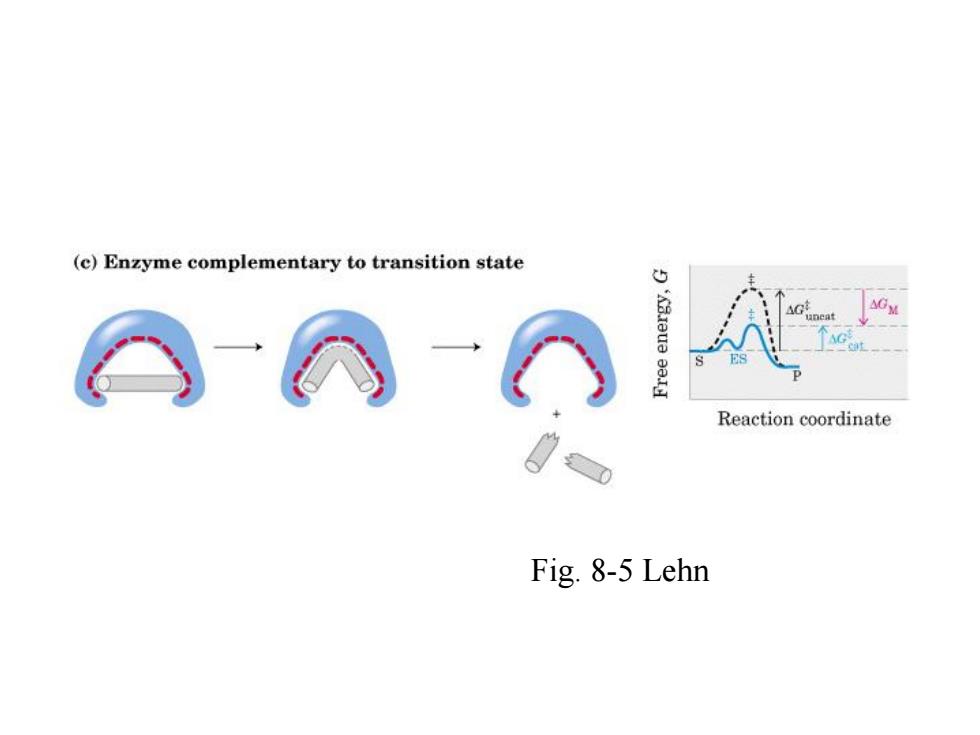
(c)Enzyme complementary to transition state G neat Reaction coordinate Fig.8-5 Lehn
Fig. 8-5 Lehn