正在加载图片...
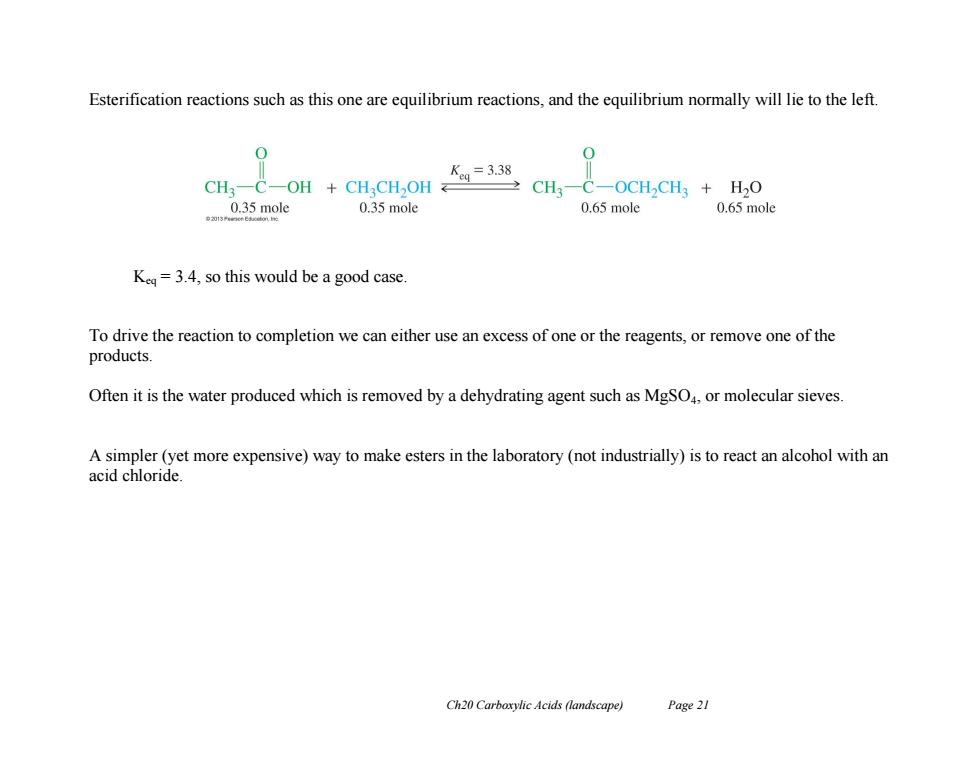
Esterification reactions such as this one are equilibrium reactions,and the equilibrium normally will lie to the left. 0 0 Keg=3.38 CH3-C- -OH CHCH,OH 2CH3一C-OCH2CH3+H20 0.35 mole 0.35 mole 0.65 mole 0.65 mole Keq=3.4,so this would be a good case. To drive the reaction to completion we can either use an excess of one or the reagents,or remove one of the products. Often it is the water produced which is removed by a dehydrating agent such as MgSO4,or molecular sieves A simpler(yet more expensive)way to make esters in the laboratory (not industrially)is to react an alcohol with an acid chloride Ch20 Carboxylic Acids(landscape) Page 21Ch20 Carboxylic Acids (landscape) Page 21 Esterification reactions such as this one are equilibrium reactions, and the equilibrium normally will lie to the left. Keq = 3.4, so this would be a good case. To drive the reaction to completion we can either use an excess of one or the reagents, or remove one of the products. Often it is the water produced which is removed by a dehydrating agent such as MgSO4, or molecular sieves. A simpler (yet more expensive) way to make esters in the laboratory (not industrially) is to react an alcohol with an acid chloride