正在加载图片...
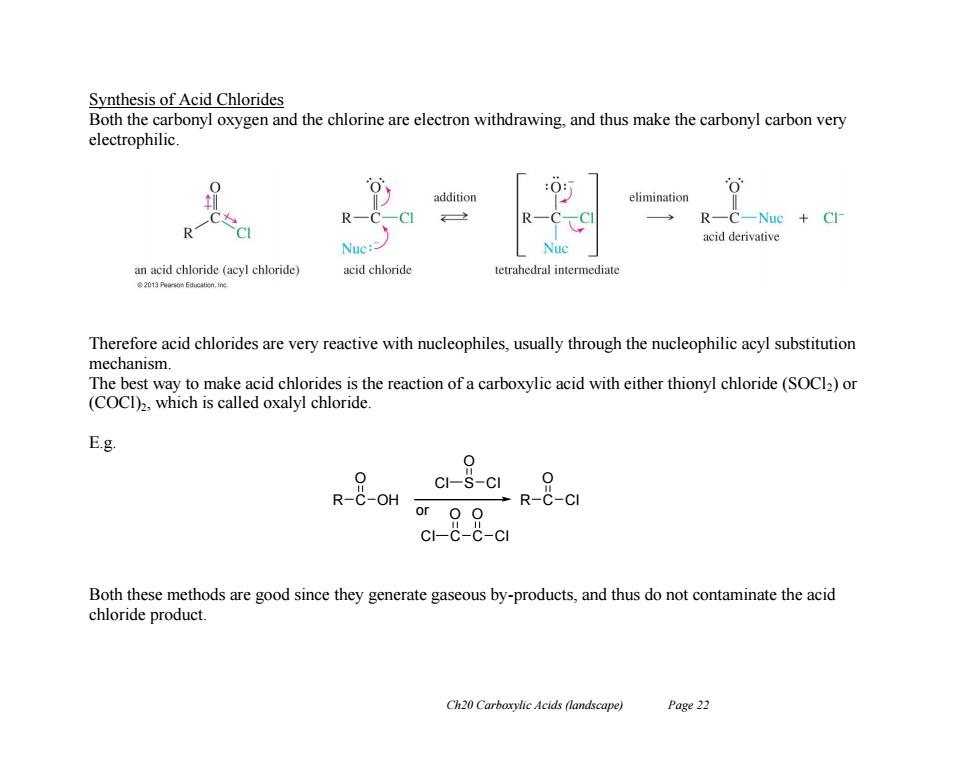
Synthesis of Acid Chlorides Both the carbonyl oxygen and the chlorine are electron withdrawing,and thus make the carbonyl carbon very electrophilic elimination R-C-Nuc Cl- R acid derivative Nuc: Nuc an acid chloride (acyl chloride) acid chloride tetrahedral intermediate Therefore acid chlorides are very reactive with nucleophiles,usually through the nucleophilic acyl substitution mechanism. The best way to make acid chlorides is the reaction of a carboxylic acid with either thionyl chloride (SOCl2)or (COCI),which is called oxalyl chloride. E.g. 0 c1-S-c0 R-C-OH →R-C-CI or OO CI-C-C-CI Both these methods are good since they generate gaseous by-products,and thus do not contaminate the acid chloride product. Ch20 Carboxylic Acids (landscape) Page 22 Ch20 Carboxylic Acids (landscape) Page 22 Synthesis of Acid Chlorides Both the carbonyl oxygen and the chlorine are electron withdrawing, and thus make the carbonyl carbon very electrophilic. Therefore acid chlorides are very reactive with nucleophiles, usually through the nucleophilic acyl substitution mechanism. The best way to make acid chlorides is the reaction of a carboxylic acid with either thionyl chloride (SOCl2) or (COCl)2, which is called oxalyl chloride. E.g. Both these methods are good since they generate gaseous by-products, and thus do not contaminate the acid chloride product. R C O OH R C O Cl Cl S O Cl or Cl C O C O Cl