正在加载图片...
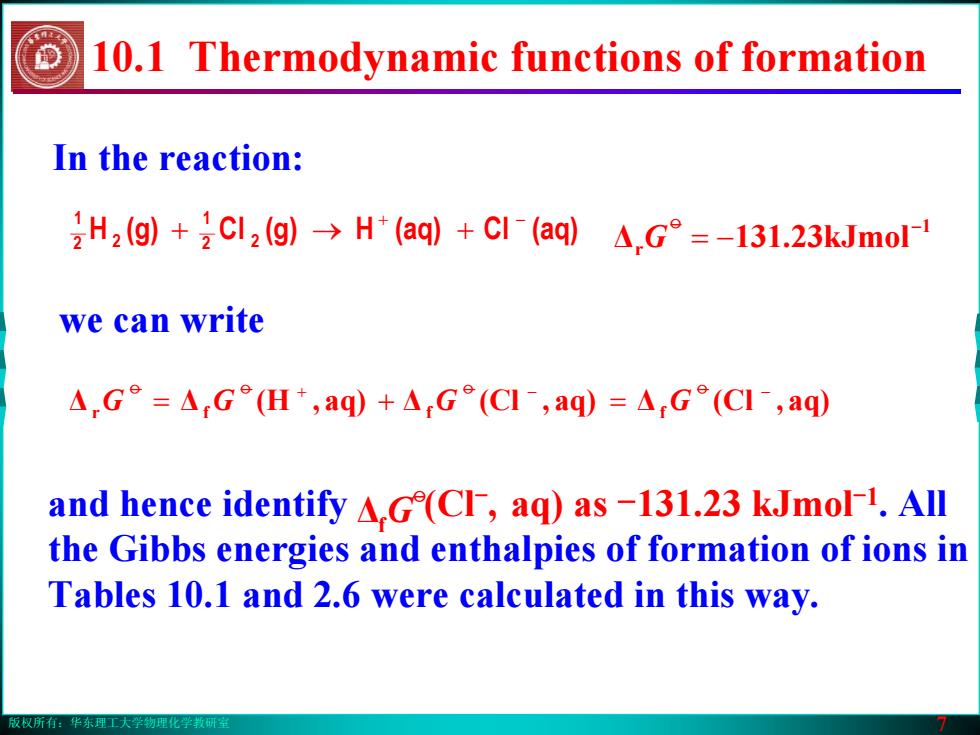
版权所有:华东理工大学物理化学教研室 7 In the reaction: 2 (aq)Cl(aq)H(g)Cl(g)H 21 2 2 1 + − + → + we can write ΔΔ aq),(H Δ aq),(Cl Δ aq),(Cl r f f f + − − = + = oo o o GG G G 1 Δr kJmol23.311 − −= o G and hence identify (Cl-, aq) as -131.23 kJmol-1. All the Gibbs energies and enthalpies of formation of ions in Tables 10.1 and 2.6 were calculated in this way. Δf o G 10.1 Thermodynamic functions of formation版权所有:华东理工大学物理化学教研室 7 In the reaction: 2 (aq)Cl(aq)H(g)Cl(g)H 21 2 2 1 + − + → + we can write ΔΔ aq),(H Δ aq),(Cl Δ aq),(Cl r f f f + − − = + = oo o o GG G G 1 Δr kJmol23.311 − −= o G and hence identify (Cl-, aq) as -131.23 kJmol-1. All the Gibbs energies and enthalpies of formation of ions in Tables 10.1 and 2.6 were calculated in this way. Δf o G 10.1 Thermodynamic functions of formation