正在加载图片...
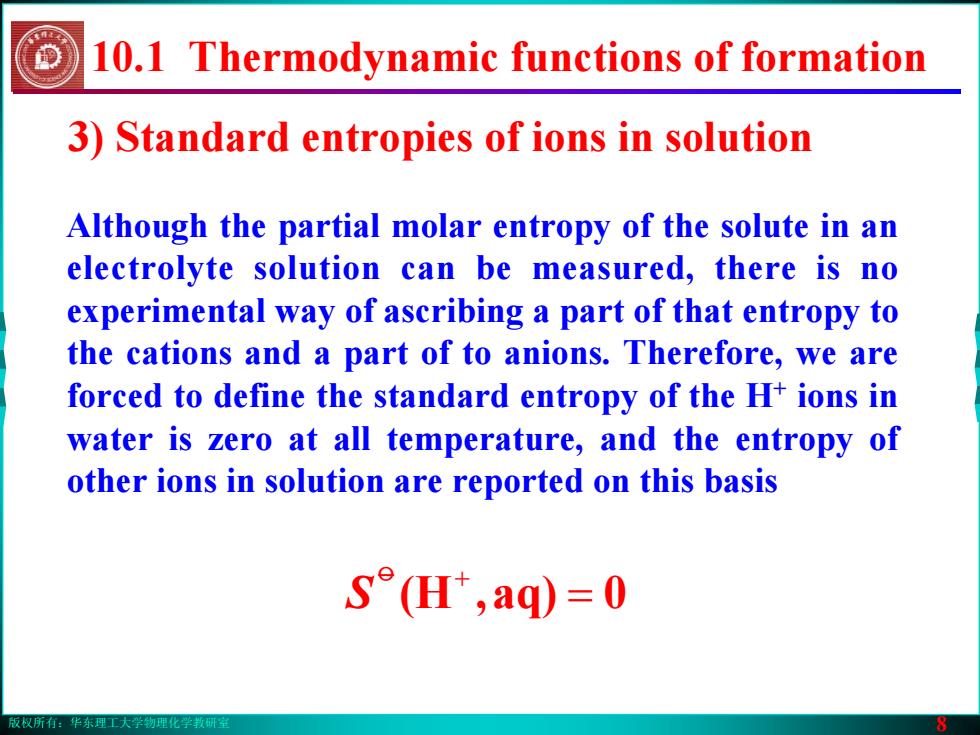
版权所有:华东理工大学物理化学教研室 8 Although the partial molar entropy of the solute in an electrolyte solution can be measured, there is no experimental way of ascribing a part of that entropy to the cations and a part of to anions. Therefore, we are forced to define the standard entropy of the H+ ions in water is zero at all temperature, and the entropy of other ions in solution are reported on this basis 3) Standard entropies of ions in solution = 0aq),(Ho + S 10.1 Thermodynamic functions of formation版权所有:华东理工大学物理化学教研室 8 Although the partial molar entropy of the solute in an electrolyte solution can be measured, there is no experimental way of ascribing a part of that entropy to the cations and a part of to anions. Therefore, we are forced to define the standard entropy of the H+ ions in water is zero at all temperature, and the entropy of other ions in solution are reported on this basis 3) Standard entropies of ions in solution = 0aq),(Ho + S 10.1 Thermodynamic functions of formation