正在加载图片...
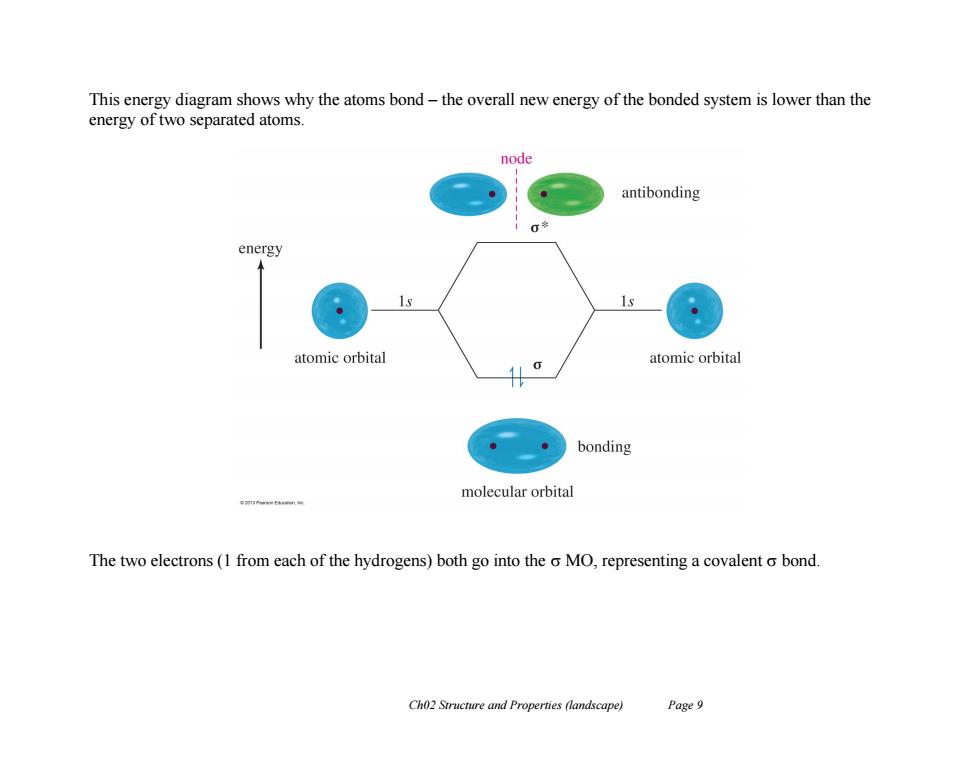
This energy diagram shows why the atoms bond-the overall new energy of the bonded system is lower than the energy of two separated atoms. node antibonding 00 energy 1s atomic orbital atomic orbital bonding molecular orbital The two electrons(1 from each of the hydrogens)both go into the o MO,representing a covalent o bond. Ch02 Structure and Properties (landscape) Page 9Ch02 Structure and Properties (landscape) Page 9 This energy diagram shows why the atoms bond – the overall new energy of the bonded system is lower than the energy of two separated atoms. The two electrons (1 from each of the hydrogens) both go into the MO, representing a covalent bond