正在加载图片...
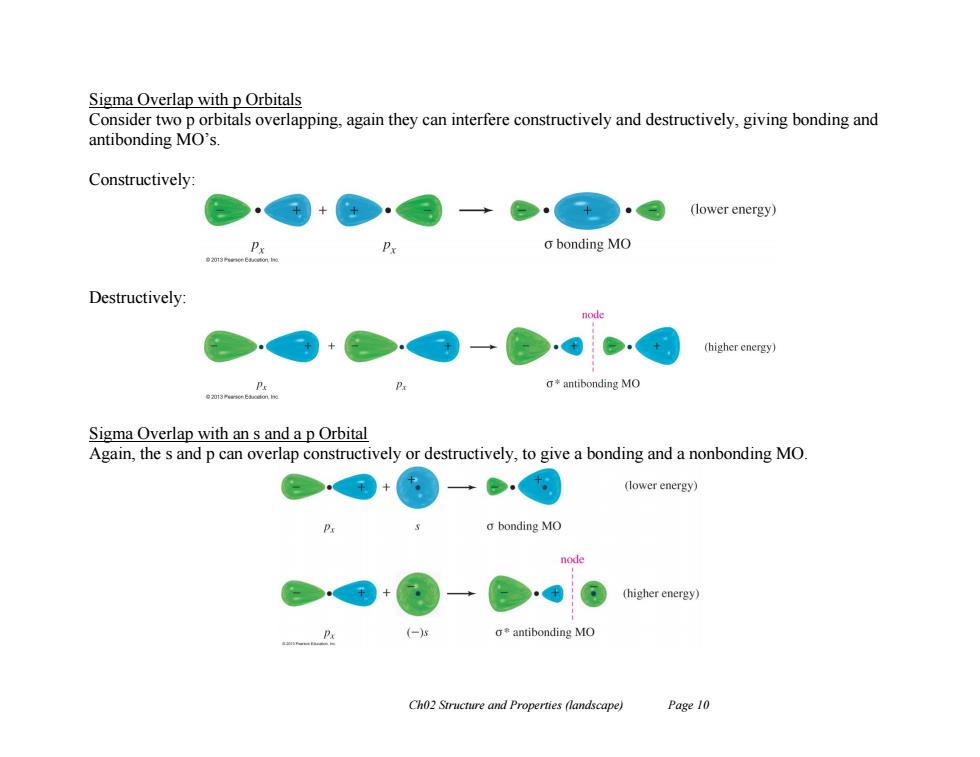
Sigma Overlap with p Orbitals Consider two p orbitals overlapping,again they can interfere constructively and destructively,giving bonding and antibonding MO's Constructively: (lower energy) o bonding MO Destructively: node (higher energy) g*antibonding MO Sigma Overlap with an s and a p Orbital Again,the s and p can overlap constructively or destructively,to give a bonding and a nonbonding MO + (lower energy) o bonding MO node (higher energy) (-) a*antibonding MO Ch02 Structure and Properties (landscape) Page 10 Ch02 Structure and Properties (landscape) Page 10 Sigma Overlap with p Orbitals Consider two p orbitals overlapping, again they can interfere constructively and destructively, giving bonding and antibonding MO’s. Constructively: Destructively: Sigma Overlap with an s and a p Orbital Again, the s and p can overlap constructively or destructively, to give a bonding and a nonbonding MO