正在加载图片...
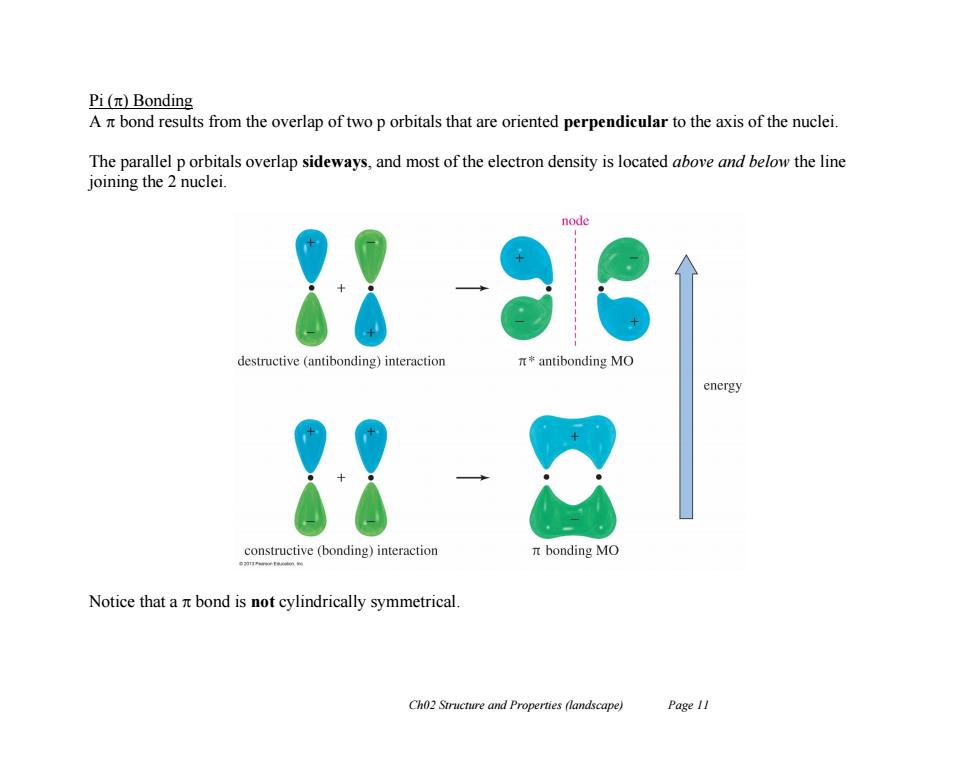
Pi(π)Bonding A bond results from the overlap of two p orbitals that are oriented perpendicular to the axis of the nuclei. The parallel p orbitals overlap sideways,and most of the electron density is located above and below the line joining the 2 nuclei. destructive (antibonding)interaction π*antibonding MO energy constructive (bonding)interaction nt bonding MO Notice that a nt bond is not cylindrically symmetrical. Ch02 Structure and Properties (landscape) Page 11Ch02 Structure and Properties (landscape) Page 11 Pi () Bonding A bond results from the overlap of two p orbitals that are oriented perpendicular to the axis of the nuclei. The parallel p orbitals overlap sideways, and most of the electron density is located above and below the line joining the 2 nuclei. Notice that a bond is not cylindrically symmetrical