正在加载图片...
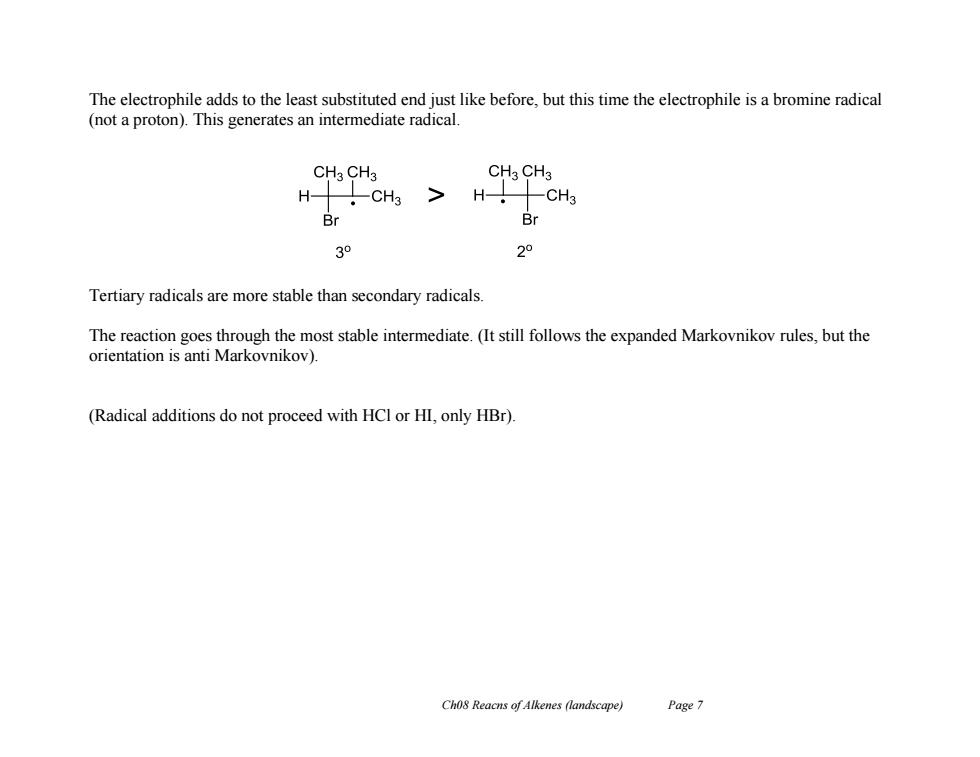
The electrophile adds to the least substituted end just like before,but this time the electrophile is a bromine radical (not a proton).This generates an intermediate radical. CH3 CH3 CH3 CH3 H H- CH3 Br 03o 2° Tertiary radicals are more stable than secondary radicals. The reaction goes through the most stable intermediate.(It still follows the expanded Markovnikov rules,but the orientation is anti Markovnikov). (Radical additions do not proceed with HCl or HI,only HBr). Ch08 Reacns of Alkenes (landscape) Page 7Ch08 Reacns of Alkenes (landscape) Page 7 The electrophile adds to the least substituted end just like before, but this time the electrophile is a bromine radical (not a proton). This generates an intermediate radical. Tertiary radicals are more stable than secondary radicals. The reaction goes through the most stable intermediate. (It still follows the expanded Markovnikov rules, but the orientation is anti Markovnikov). (Radical additions do not proceed with HCl or HI, only HBr)