正在加载图片...
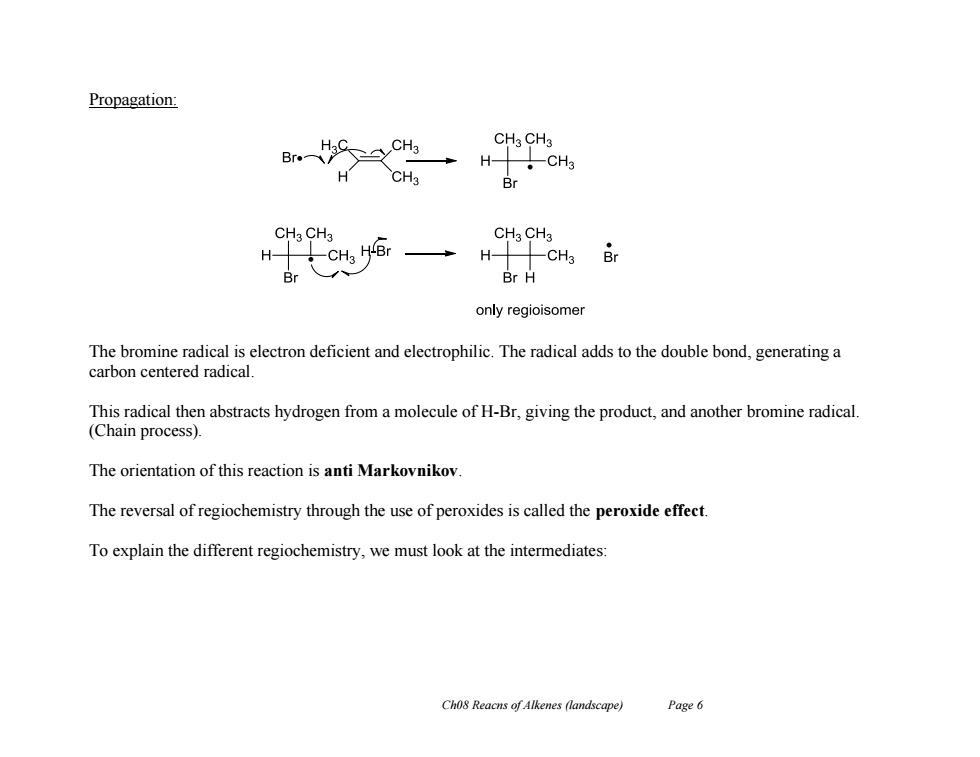
Propagation: CHa CH3 Br H CH3 H-11CH Br CHa CH3 CHaCH3 H十CH 5 Br Br H only regioisomer The bromine radical is electron deficient and electrophilic.The radical adds to the double bond,generating a carbon centered radical. This radical then abstracts hydrogen from a molecule of H-Br,giving the product,and another bromine radical. (Chain process). The orientation of this reaction is anti Markovnikov The reversal of regiochemistry through the use of peroxides is called the peroxide effect. To explain the different regiochemistry,we must look at the intermediates: Ch08 Reacns of Alkenes (landscape) Page 6 Ch08 Reacns of Alkenes (landscape) Page 6 Propagation: The bromine radical is electron deficient and electrophilic. The radical adds to the double bond, generating a carbon centered radical. This radical then abstracts hydrogen from a molecule of H-Br, giving the product, and another bromine radical. (Chain process). The orientation of this reaction is anti Markovnikov. The reversal of regiochemistry through the use of peroxides is called the peroxide effect. To explain the different regiochemistry, we must look at the intermediates: