正在加载图片...
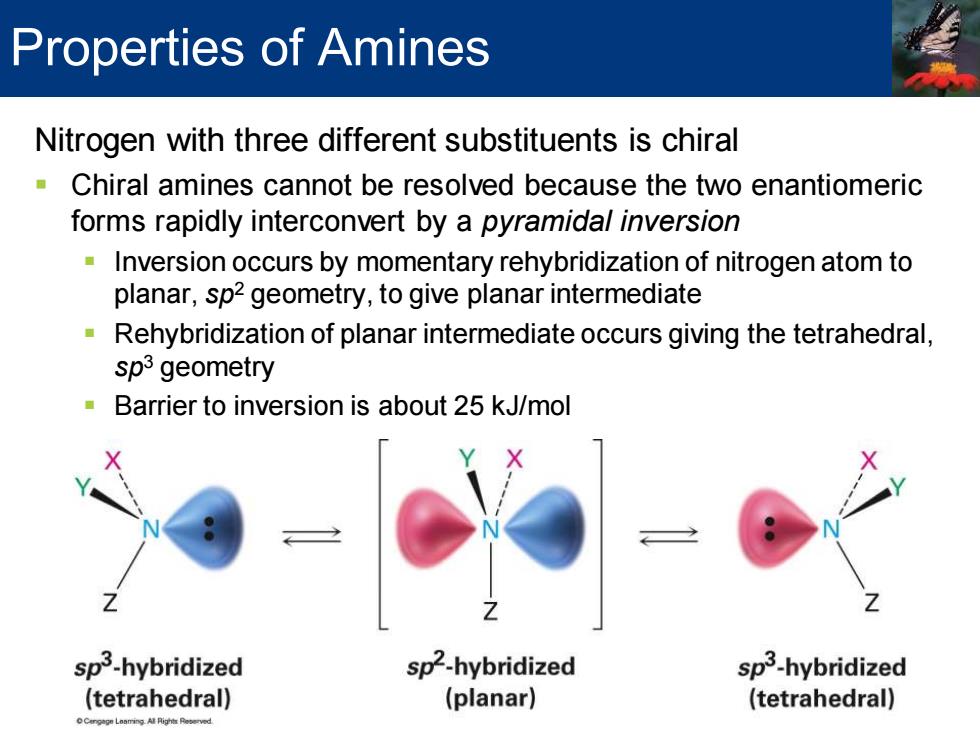
Properties of Amines Nitrogen with three different substituents is chiral Chiral amines cannot be resolved because the two enantiomeric forms rapidly interconvert by a pyramidal inversion Inversion occurs by momentary rehybridization of nitrogen atom to planar,sp2 geometry,to give planar intermediate Rehybridization of planar intermediate occurs giving the tetrahedral, sp3 geometry Barrier to inversion is about 25 kJ/mol sp3-hybridized sp2-hybridized sp3-hybridized (tetrahedral) (planar) (tetrahedral)Nitrogen with three different substituents is chiral ▪ Chiral amines cannot be resolved because the two enantiomeric forms rapidly interconvert by a pyramidal inversion ▪ Inversion occurs by momentary rehybridization of nitrogen atom to planar, sp2 geometry, to give planar intermediate ▪ Rehybridization of planar intermediate occurs giving the tetrahedral, sp3 geometry ▪ Barrier to inversion is about 25 kJ/mol Properties of Amines