正在加载图片...
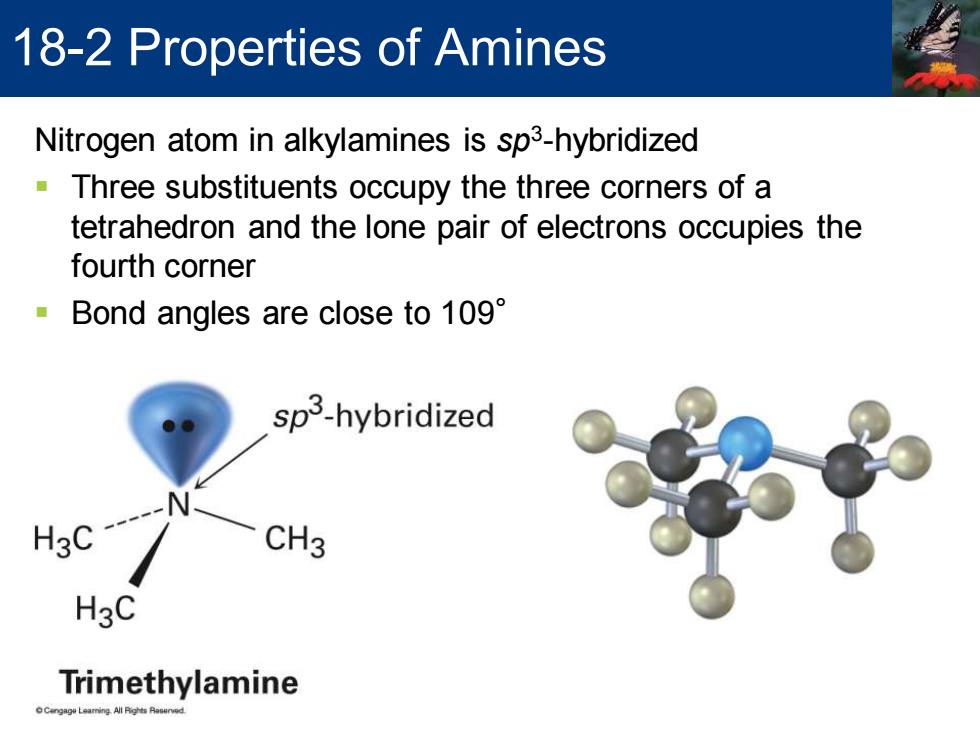
18-2 Properties of Amines Nitrogen atom in alkylamines is sp3-hybridized Three substituents occupy the three corners of a tetrahedron and the lone pair of electrons occupies the fourth corner Bond angles are close to 109 sp3-hybridized HgC CH3 H3C Trimethylamine Nitrogen atom in alkylamines is sp3 -hybridized ▪ Three substituents occupy the three corners of a tetrahedron and the lone pair of electrons occupies the fourth corner ▪ Bond angles are close to 109° 18-2 Properties of Amines