正在加载图片...
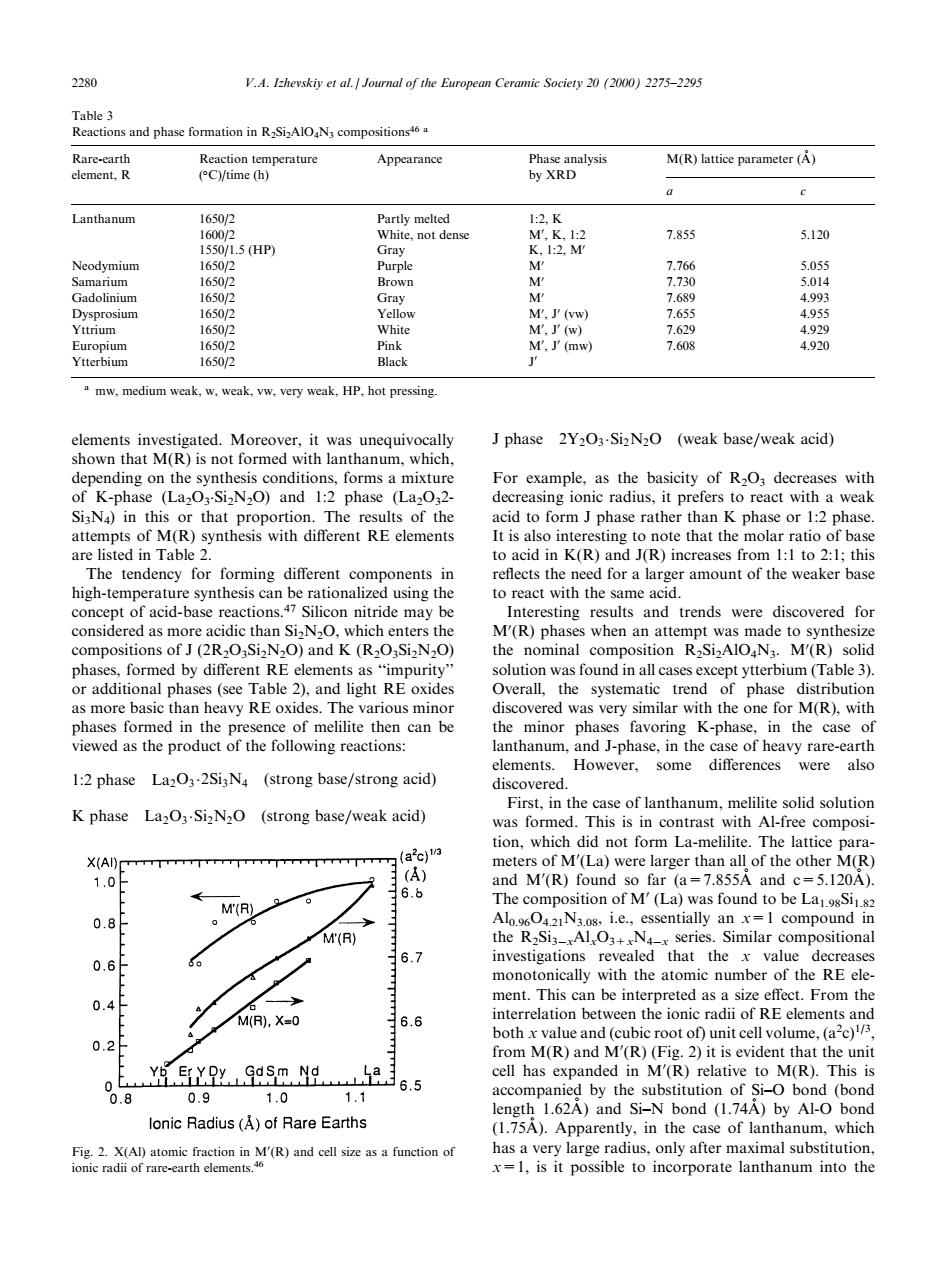
2280 V.A.Ihevskiy et al Jounal of the European Ceramic Society2(20)275-2295 Appearance y XBD M(R)lattice parameter (A) Lanthanum 7.855 5.120 HP doliniur M.) mw.medium weak.w.weak.vw.very weak.HP,hot pressing shownndo ored with thny J phase 2Y2O3-Si2N2O (weak base/weak acid) own anthanum,which of K-phase (LaO-SizN2O)and 1:2 phase (La0.2. SisN)in this or that proportion.The results of the acid to form J phase rather than K phase or 1:2 phase aThe tende for forming differen ed for a high-temperature synthesis can be rationalized using the to react with the same acid. rie may be 020SN-0andK )pr inal phases.formed by different RE elements asimpurity or additional phases(see Table 2),and light RE oxides as more basic than heavy RE oxides. discovered w s very similar wi the one for elements.However,some differences were also 1:2 phase La2O3-2SiN (strong base/strong acid) K phase La2O3-SizN2O (strong base/weak acid) numt-fr melilite solid solutior tion,which did not form La-melilite.The lattice para 11/ X(AI) 1.0 (A) A 6.b 0.8 。MA M(R) the R2Siz_Al. series.Similar compositional 0.6 6.7 the valu he nt Thi M.X=0 interrelation between the ionic radii of RE elements and 6.6 0.2f bothvalue and (cubic root of)unit cell volume.(ac) o(R)ad M(R)T 65 ompanie 1.0 1.1 d by the substitution of Si-O bond (bond 0.9 1.62A)and Si-N bond (1.74A)by Al-O bond lonic Radius(A)of Rare Earths anum whicelements investigated. Moreover, it was unequivocally shown that M(R) is not formed with lanthanum, which, depending on the synthesis conditions, forms a mixture of K-phase (La2O3 .Si2N2O) and 1:2 phase (La2O32- Si3N4) in this or that proportion. The results of the attempts of M(R) synthesis with dierent RE elements are listed in Table 2. The tendency for forming dierent components in high-temperature synthesis can be rationalized using the concept of acid-base reactions.47 Silicon nitride may be considered as more acidic than Si2N2O, which enters the compositions of J (2R2O3Si2N2O) and K (R2O3Si2N2O) phases, formed by dierent RE elements as ``impurity'' or additional phases (see Table 2), and light RE oxides as more basic than heavy RE oxides. The various minor phases formed in the presence of melilite then can be viewed as the product of the following reactions: 1:2 phase La2O3 2Si3N4
strong base=strong acid K phase La2O3 Si2N2O
strong base=weak acid J phase 2Y2O3 Si2N2O
weak base=weak acid For example, as the basicity of R2O3 decreases with decreasing ionic radius, it prefers to react with a weak acid to form J phase rather than K phase or 1:2 phase. It is also interesting to note that the molar ratio of base to acid in K(R) and J(R) increases from 1:1 to 2:1; this re¯ects the need for a larger amount of the weaker base to react with the same acid. Interesting results and trends were discovered for M0 (R) phases when an attempt was made to synthesize the nominal composition R2Si2AlO4N3. M0 (R) solid solution was found in all cases except ytterbium (Table 3). Overall, the systematic trend of phase distribution discovered was very similar with the one for M(R), with the minor phases favoring K-phase, in the case of lanthanum, and J-phase, in the case of heavy rare-earth elements. However, some dierences were also discovered. First, in the case of lanthanum, melilite solid solution was formed. This is in contrast with Al-free composition, which did not form La-melilite. The lattice parameters of M0 (La) were larger than all of the other M(R) and M0 (R) found so far (a=7.855A and c=5.120A ). The composition of M0 (La) was found to be La1.98Si1.82 Al0.96O4.21N3.08, i.e., essentially an x=1 compound in the R2Si3ÿxAlxO3+xN4ÿx series. Similar compositional investigations revealed that the x value decreases monotonically with the atomic number of the RE element. This can be interpreted as a size eect. From the interrelation between the ionic radii of RE elements and both x value and (cubic root of) unit cell volume, (a2 c)1/3, from M(R) and M0 (R) (Fig. 2) it is evident that the unit cell has expanded in M0 (R) relative to M(R). This is accompanied by the substitution of Si±O bond (bond length 1.62A ) and Si±N bond (1.74AÊ ) by Al-O bond (1.75AÊ ). Apparently, in the case of lanthanum, which has a very large radius, only after maximal substitution, x=1, is it possible to incorporate lanthanum into the Table 3 Reactions and phase formation in R2Si2AlO4N3 compositions46 a Rare-earth element, R Reaction temperature ( C)/time (h) Appearance Phase analysis by XRD M(R) lattice parameter (A ) a c Lanthanum 1650/2 Partly melted 1:2, K 1600/2 White, not dense M0 , K, 1:2 7.855 5.120 1550/1.5 (HP) Gray K, 1:2, M0 Neodymium 1650/2 Purple M0 7.766 5.055 Samarium 1650/2 Brown M0 7.730 5.014 Gadolinium 1650/2 Gray M0 7.689 4.993 Dysprosium 1650/2 Yellow M0 , J0 (vw) 7.655 4.955 Yttrium 1650/2 White M0 , J0 (w) 7.629 4.929 Europium 1650/2 Pink M0 , J0 (mw) 7.608 4.920 Ytterbium 1650/2 Black J0 a mw, medium weak, w, weak, vw, very weak, HP, hot pressing. Fig. 2. X(Al) atomic fraction in M0 (R) and cell size as a function of ionic radii of rare-earth elements.46 2280 V.A. Izhevskiy et al. / Journal of the European Ceramic Society 20 (2000) 2275±2295