正在加载图片...
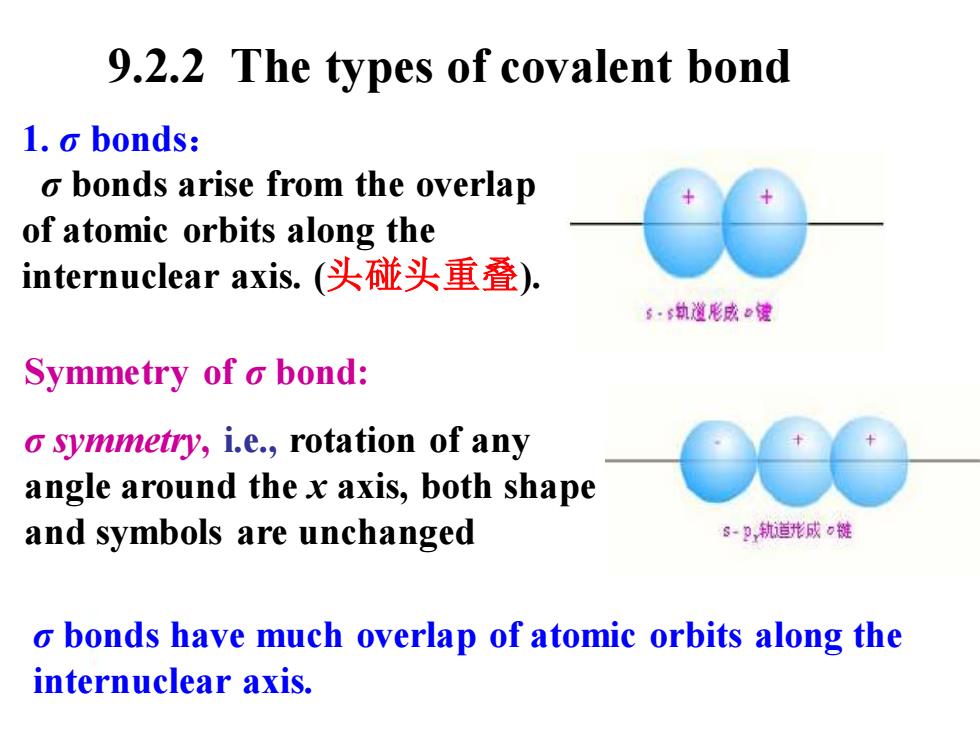
9.2.2 The types of covalent bond 1.o bonds: o bonds arise from the overlap of atomic orbits along the internuclear axis..(头碰头重叠). s·6执道形成e健 Symmetry of o bond: o symmetry,i.e.,rotation of any angle around the x axis,both shape and symbols are unchanged s-P轨道形成0链 o bonds have much overlap of atomic orbits along the internuclear axis. 1. σ bonds: σ bonds arise from the overlap of atomic orbits along the internuclear axis. (头碰头重叠). Symmetry of σ bond: σ symmetry, i.e., rotation of any angle around the x axis, both shape and symbols are unchanged σ bonds have much overlap of atomic orbits along the internuclear axis. 9.2.2 The types of covalent bond