正在加载图片...
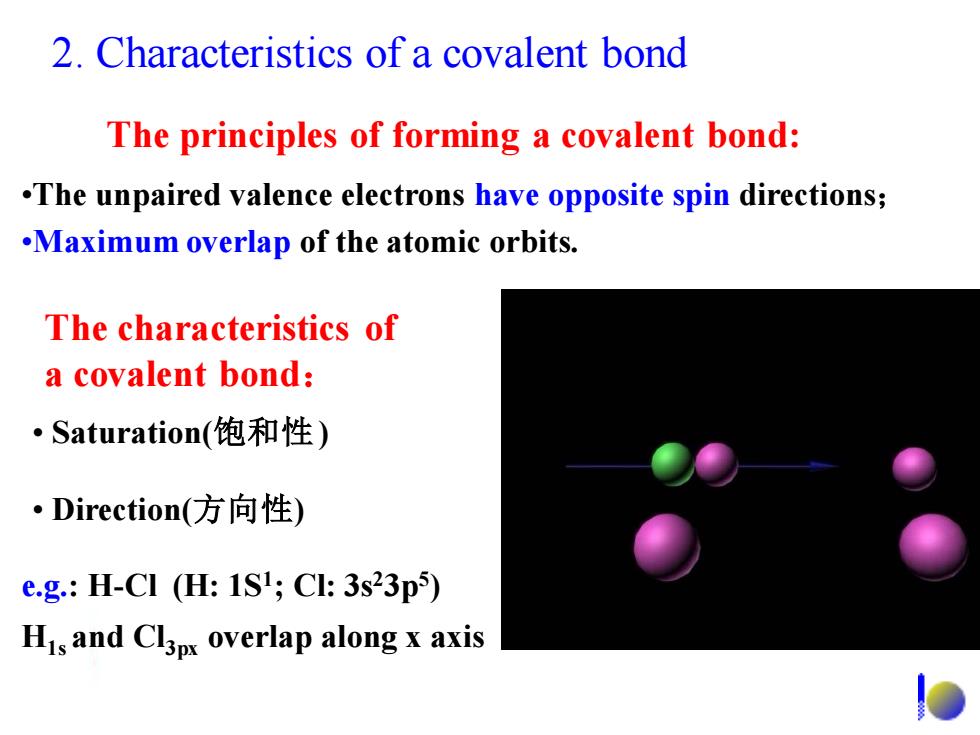
2.Characteristics of a covalent bond The principles of forming a covalent bond: .The unpaired valence electrons have opposite spin directions; .Maximum overlap of the atomic orbits. The characteristics of a covalent bond: Saturation(饱和性) ·Direction(方向性) e.g.:H-CI (H:1SI;Cl:3s23p5) His and Cl3px overlap along x axis2. Characteristics of a covalent bond The principles of forming a covalent bond: •The unpaired valence electrons have opposite spin directions; •Maximum overlap of the atomic orbits. The characteristics of a covalent bond: • Direction(方向性) • Saturation(饱和性) e.g.: H-Cl (H: 1S1 ; Cl: 3s23p5 ) H1s and Cl3px overlap along x axis