正在加载图片...
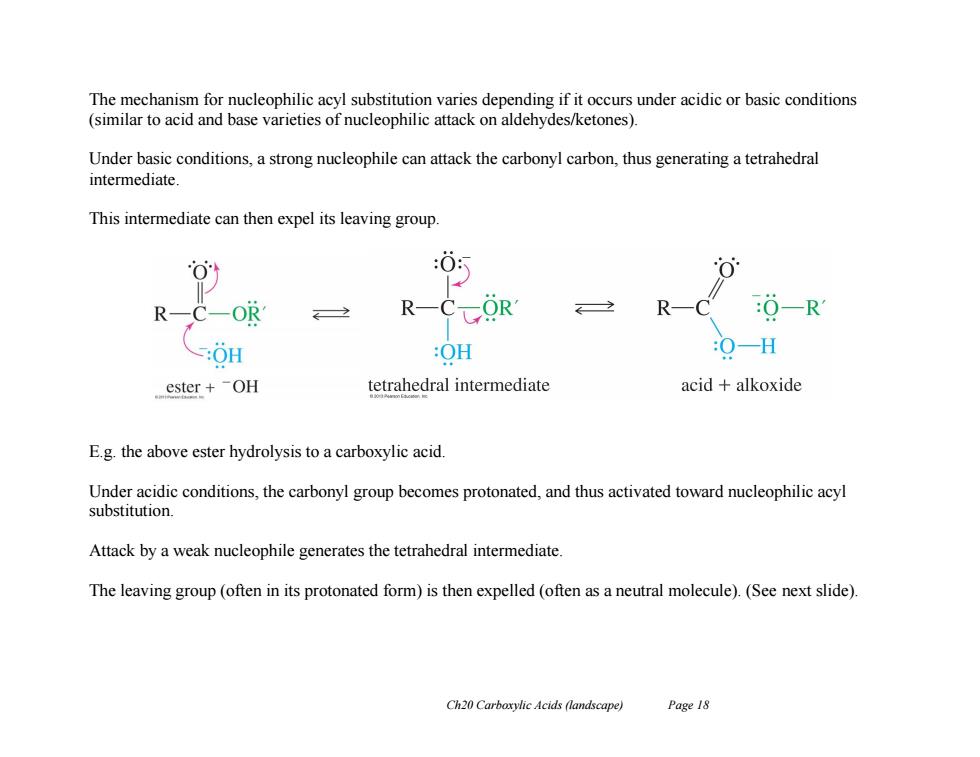
The mechanism for nucleophilic acyl substitution varies depending if it occurs under acidic or basic conditions (similar to acid and base varieties of nucleophilic attack on aldehydes/ketones). Under basic conditions,a strong nucleophile can attack the carbonyl carbon,thus generating a tetrahedral intermediate This intermediate can then expel its leaving group. :0: OR R-C- OR :O-R :OH :OH 0一H ester+OH tetrahedral intermediate acid alkoxide E.g.the above ester hydrolysis to a carboxylic acid. Under acidic conditions,the carbonyl group becomes protonated,and thus activated toward nucleophilic acyl substitution. Attack by a weak nucleophile generates the tetrahedral intermediate. The leaving group(often in its protonated form)is then expelled (often as a neutral molecule).(See next slide). Ch20 Carboxylic Acids (landscape) Page 18 Ch20 Carboxylic Acids (landscape) Page 18 The mechanism for nucleophilic acyl substitution varies depending if it occurs under acidic or basic conditions (similar to acid and base varieties of nucleophilic attack on aldehydes/ketones). Under basic conditions, a strong nucleophile can attack the carbonyl carbon, thus generating a tetrahedral intermediate. This intermediate can then expel its leaving group. E.g. the above ester hydrolysis to a carboxylic acid. Under acidic conditions, the carbonyl group becomes protonated, and thus activated toward nucleophilic acyl substitution. Attack by a weak nucleophile generates the tetrahedral intermediate. The leaving group (often in its protonated form) is then expelled (often as a neutral molecule). (See next slide)