正在加载图片...
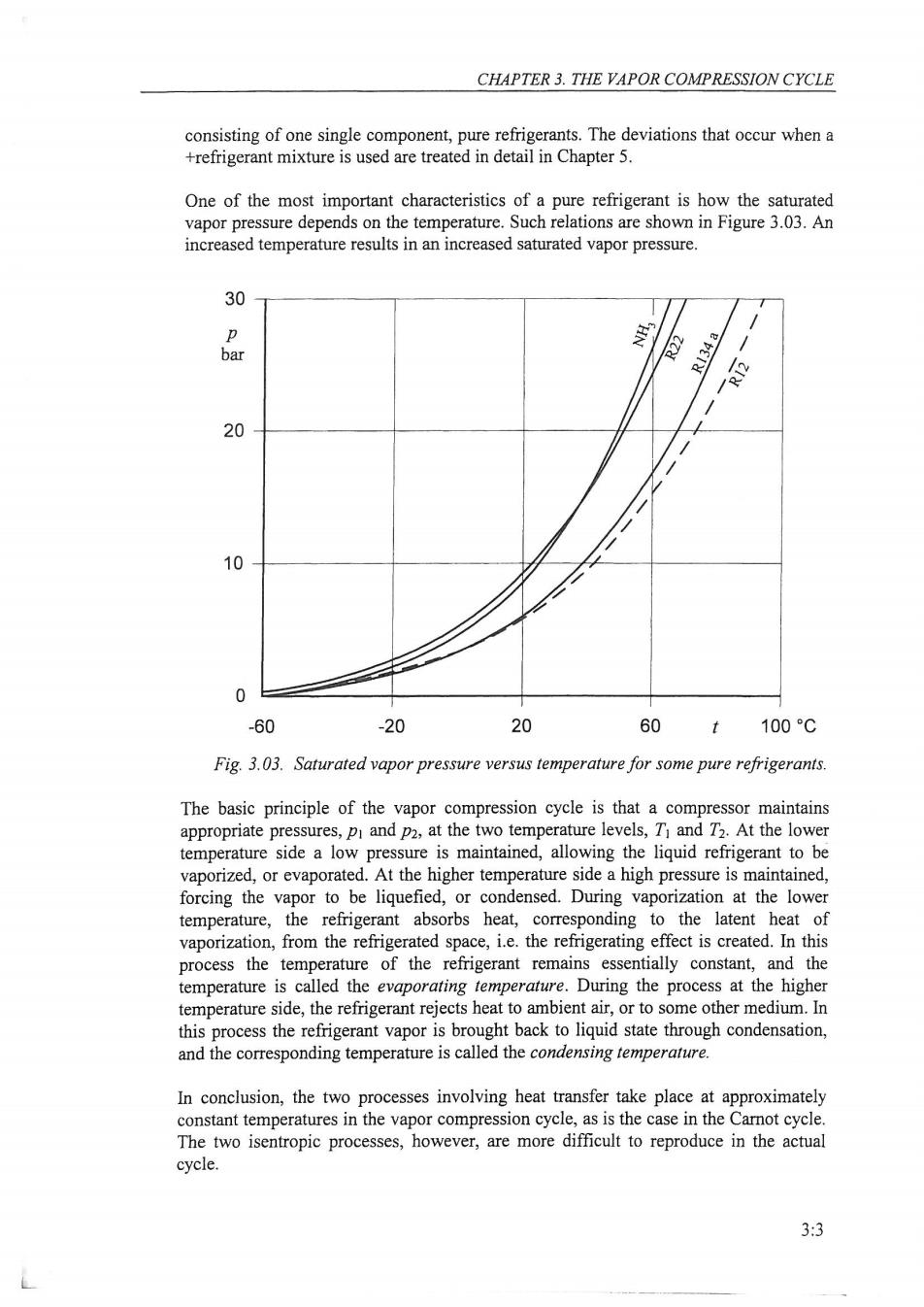
CHAPTER 3.THE VAPOR COMPRESSION CYCLE consisting of one single component,pure refrigerants.The deviations that occur when a +refrigerant mixture is used are treated in detail in Chapter 5. One of the most important characteristics of a pure refrigerant is how the saturated vapor pressure depends on the temperature.Such relations are shown in Figure 3.03.An increased temperature results in an increased saturated vapor pressure. 30 p NH: bar R134a e 20 10 0 -60 -20 20 60 t 100C Fig.3.03.Saturated vapor pressure versus temperature for some pure refrigerants. The basic principle of the vapor compression cycle is that a compressor maintains appropriate pressures,pi and p2,at the two temperature levels,T and T2.At the lower temperature side a low pressure is maintained,allowing the liquid refrigerant to be vaporized,or evaporated.At the higher temperature side a high pressure is maintained, forcing the vapor to be liquefied,or condensed.During vaporization at the lower temperature,the refrigerant absorbs heat,corresponding to the latent heat of vaporization,from the refrigerated space,i.e.the refrigerating effect is created.In this process the temperature of the refrigerant remains essentially constant,and the temperature is called the evaporating temperature.During the process at the higher temperature side,the refrigerant rejects heat to ambient air,or to some other medium.In this process the refrigerant vapor is brought back to liquid state through condensation, and the corresponding temperature is called the condensing temperature. In conclusion,the two processes involving heat transfer take place at approximately constant temperatures in the vapor compression cycle,as is the case in the Carnot cycle. The two isentropic processes,however,are more difficult to reproduce in the actual cycle. 3:3