正在加载图片...
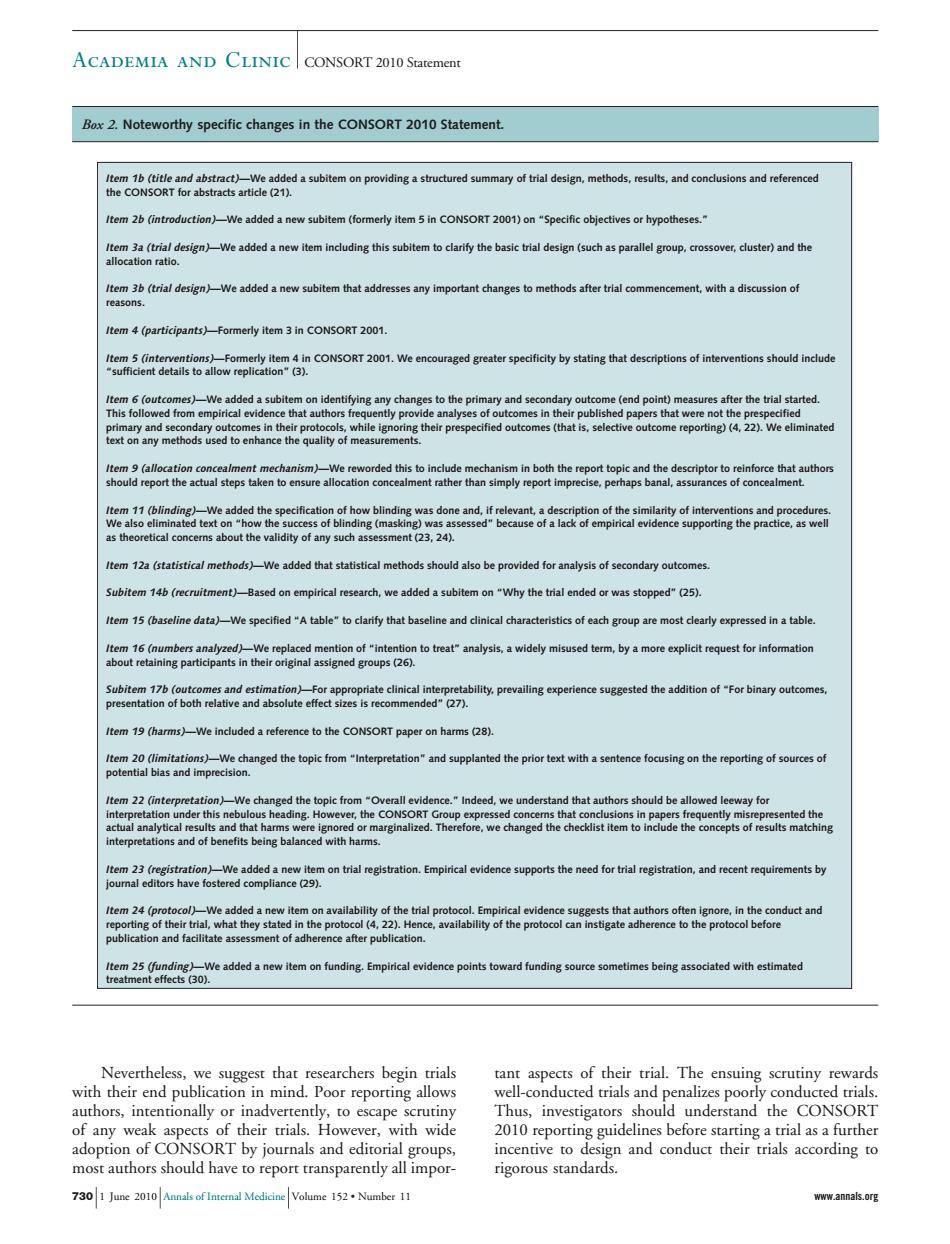
ACADEMIA AND CLINIC CONSORT 2010 Statement .Noteworthy specific changes in the Statement. eatntttdabtemonpodiasatnctredsunmayodnldegn,mehod4,eub,sndconcdn s and reference em 2b (in e added anew er dusted)and th tem 36(trial design)We added a new subitem that addresses any important changes to methods after trial co ment.with a discussion of Item4(participants)Fommerly. ntions should incluc d a sub m on identitying any cha da out the tem 14b(recruitment)-Based on empirical research,we added a subitem on "Why the trial ended or was stopped"(25) ifiedtable"to clarify that baseline and tem 19 (harms)-We included a reference to the CONSORT paper on harms 28) We change autho ed leeway to that h e ignored e after publication ociated with estimated scarchers begin trial s of their trial The ensu authors,intentionally or inadvertently,to escape scrutiny and ec n7wid Thus,investigators should understand the CONSORT should h all groups. 7301 June 201Amnals ofInternal Medicine Volume 152Number 11 www.annals.org Nevertheless, we suggest that researchers begin trials with their end publication in mind. Poor reporting allows authors, intentionally or inadvertently, to escape scrutiny of any weak aspects of their trials. However, with wide adoption of CONSORT by journals and editorial groups, most authors should have to report transparently all important aspects of their trial. The ensuing scrutiny rewards well-conducted trials and penalizes poorly conducted trials. Thus, investigators should understand the CONSORT 2010 reporting guidelines before starting a trial as a further incentive to design and conduct their trials according to rigorous standards. Box 2. Noteworthy specific changes in the CONSORT 2010 Statement. Item 1b (title and abstract)—We added a subitem on providing a structured summary of trial design, methods, results, and conclusions and referenced the CONSORT for abstracts article (21). Item 2b (introduction)—We added a new subitem (formerly item 5 in CONSORT 2001) on “Specific objectives or hypotheses.” Item 3a (trial design)—We added a new item including this subitem to clarify the basic trial design (such as parallel group, crossover, cluster) and the allocation ratio. Item 3b (trial design)—We added a new subitem that addresses any important changes to methods after trial commencement, with a discussion of reasons. Item 4 (participants)—Formerly item 3 in CONSORT 2001. Item 5 (interventions)—Formerly item 4 in CONSORT 2001. We encouraged greater specificity by stating that descriptions of interventions should include “sufficient details to allow replication” (3). Item 6 (outcomes)—We added a subitem on identifying any changes to the primary and secondary outcome (end point) measures after the trial started. This followed from empirical evidence that authors frequently provide analyses of outcomes in their published papers that were not the prespecified primary and secondary outcomes in their protocols, while ignoring their prespecified outcomes (that is, selective outcome reporting) (4, 22). We eliminated text on any methods used to enhance the quality of measurements. Item 9 (allocation concealment mechanism)—We reworded this to include mechanism in both the report topic and the descriptor to reinforce that authors should report the actual steps taken to ensure allocation concealment rather than simply report imprecise, perhaps banal, assurances of concealment. Item 11 (blinding)—We added the specification of how blinding was done and, if relevant, a description of the similarity of interventions and procedures. We also eliminated text on “how the success of blinding (masking) was assessed” because of a lack of empirical evidence supporting the practice, as well as theoretical concerns about the validity of any such assessment (23, 24). Item 12a (statistical methods)—We added that statistical methods should also be provided for analysis of secondary outcomes. Subitem 14b (recruitment)—Based on empirical research, we added a subitem on “Why the trial ended or was stopped” (25). Item 15 (baseline data)—We specified “A table” to clarify that baseline and clinical characteristics of each group are most clearly expressed in a table. Item 16 (numbers analyzed)—We replaced mention of “intention to treat” analysis, a widely misused term, by a more explicit request for information about retaining participants in their original assigned groups (26). Subitem 17b (outcomes and estimation)—For appropriate clinical interpretability, prevailing experience suggested the addition of “For binary outcomes, presentation of both relative and absolute effect sizes is recommended” (27). Item 19 (harms)—We included a reference to the CONSORT paper on harms (28). Item 20 (limitations)—We changed the topic from “Interpretation” and supplanted the prior text with a sentence focusing on the reporting of sources of potential bias and imprecision. Item 22 (interpretation)—We changed the topic from “Overall evidence.” Indeed, we understand that authors should be allowed leeway for interpretation under this nebulous heading. However, the CONSORT Group expressed concerns that conclusions in papers frequently misrepresented the actual analytical results and that harms were ignored or marginalized. Therefore, we changed the checklist item to include the concepts of results matching interpretations and of benefits being balanced with harms. Item 23 (registration)—We added a new item on trial registration. Empirical evidence supports the need for trial registration, and recent requirements by journal editors have fostered compliance (29). Item 24 (protocol)—We added a new item on availability of the trial protocol. Empirical evidence suggests that authors often ignore, in the conduct and reporting of their trial, what they stated in the protocol (4, 22). Hence, availability of the protocol can instigate adherence to the protocol before publication and facilitate assessment of adherence after publication. Item 25 (funding)—We added a new item on funding. Empirical evidence points toward funding source sometimes being associated with estimated treatment effects (30). Academia and Clinic CONSORT 2010 Statement 730 1 June 2010 Annals of Internal Medicine Volume 152 • Number 11 www.annals.org