正在加载图片...
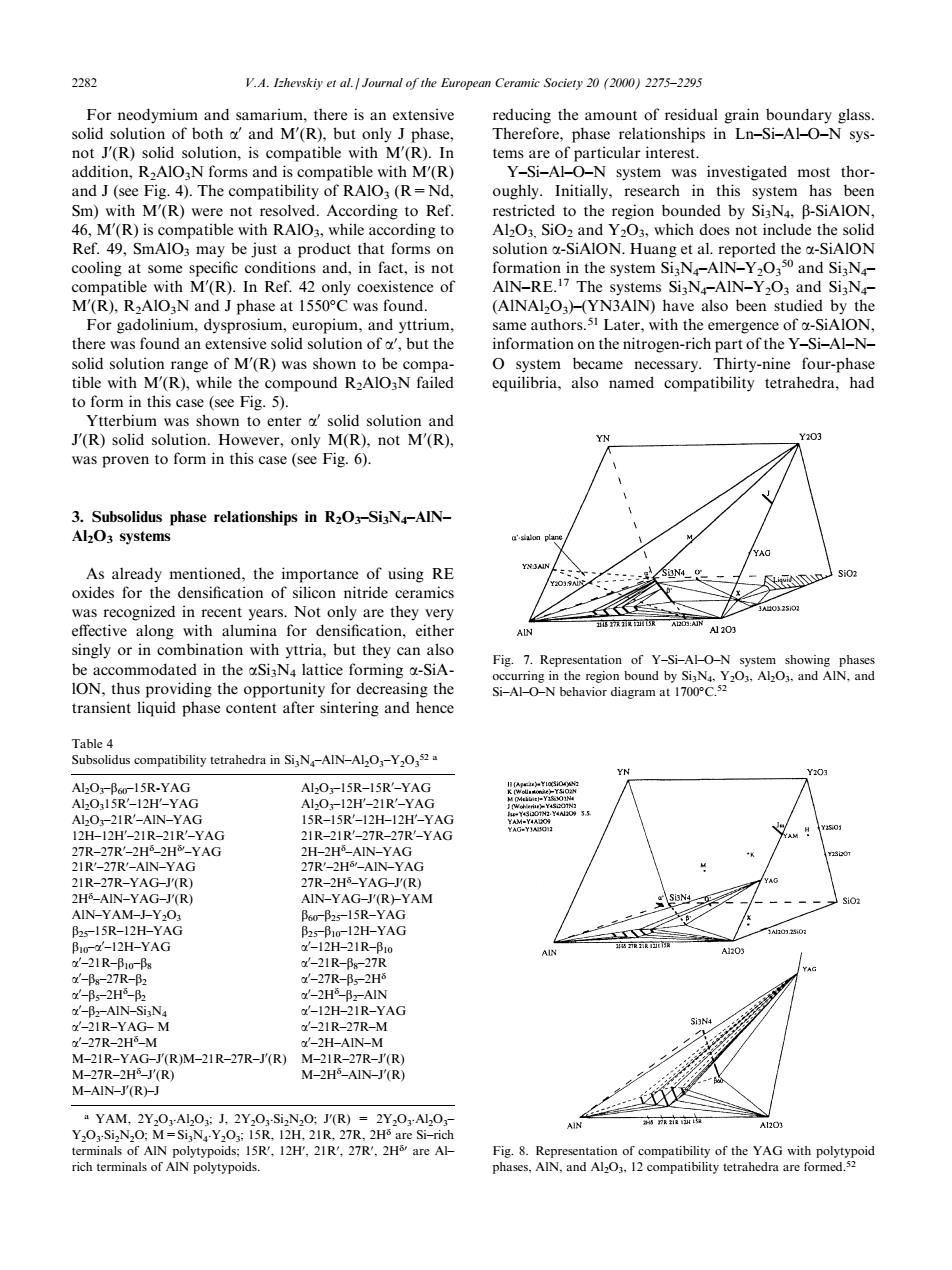
2 V.A.Izhevskiy et al.Journal of the European Ce ic Societ2012000)2275-2295 not (R)solid solution Y-Si-ALO-N system was investigated most thor and J(see Fig.4).The compatibility of RAlO,(R-Nd oughly.Initially,research in this system has beer on bounded by SiN.B Ret.49.SmAlo may be iust a product that forms on solution SiAlON.Huang et al.reported the -SiAlON le() conditions and,in fact,is not ormation in the system Si,N-AIN-Yand Si,N (R) e o SI:N ied b for gadolinium dysnrosium euronium and vttrium same authorss Later.with the emer ence of a-SiAlON there was found an extensive solid solution of a.but the information on the nitrogen-rich part of the Y-Si-Al-N- 3.Subsolidus phase relationships in R2O-Si3N-AIN- AlO systems a'siog As already mer of using Re or ong be a ommodated in the SiN latuice forming-SiA Fig.7.Re of Y-Si-AHO-N ION,thus providing the opportunity for decreasing the O-NA transient liquid phase content after sintering and hence scompatibility tetrahedra in SiN-AIN-AlO-Y AlO-Bur-15R-YAG YAC 2IR-27R-YAC 5R- YAG -21R- -2 AG 2IR-YAG-M -2IR-27R-M M2IR-YAG-T(R)M-21R-27R-J(R)M-2IR-27 R M-AIN-FRD(R) M-2H-AIN-J'(R)For neodymium and samarium, there is an extensive solid solution of both a0 and M0 (R), but only J phase, not J0 (R) solid solution, is compatible with M0 (R). In addition, R2AlO3N forms and is compatible with M0 (R) and J (see Fig. 4). The compatibility of RAlO3 (R=Nd, Sm) with M0 (R) were not resolved. According to Ref. 46, M0 (R) is compatible with RAlO3, while according to Ref. 49, SmAlO3 may be just a product that forms on cooling at some speci®c conditions and, in fact, is not compatible with M0 (R). In Ref. 42 only coexistence of M0 (R), R2AlO3N and J phase at 1550C was found. For gadolinium, dysprosium, europium, and yttrium, there was found an extensive solid solution of a0 , but the solid solution range of M0 (R) was shown to be compatible with M0 (R), while the compound R2AlO3N failed to form in this case (see Fig. 5). Ytterbium was shown to enter a0 solid solution and J0 (R) solid solution. However, only M(R), not M0 (R), was proven to form in this case (see Fig. 6). 3. Subsolidus phase relationships in R2O3±Si3N4±AlN± Al2O3 systems As already mentioned, the importance of using RE oxides for the densi®cation of silicon nitride ceramics was recognized in recent years. Not only are they very eective along with alumina for densi®cation, either singly or in combination with yttria, but they can also be accommodated in the aSi3N4 lattice forming a-SiAlON, thus providing the opportunity for decreasing the transient liquid phase content after sintering and hence reducing the amount of residual grain boundary glass. Therefore, phase relationships in Ln±Si±Al±O±N systems are of particular interest. Y±Si±Al±O±N system was investigated most thoroughly. Initially, research in this system has been restricted to the region bounded by Si3N4, b-SiAlON, Al2O3, SiO2 and Y2O3, which does not include the solid solution a-SiAlON. Huang et al. reported the a-SiAlON formation in the system Si3N4±AlN±Y2O3 50 and Si3N4± AlN±RE.17 The systems Si3N4±AlN±Y2O3 and Si3N4± (AlNAl2O3)±(YN3AlN) have also been studied by the same authors.51 Later, with the emergence of a-SiAlON, information on the nitrogen-rich part of the Y±Si±Al±N± O system became necessary. Thirty-nine four-phase equilibria, also named compatibility tetrahedra, had Table 4 Subsolidus compatibility tetrahedra in Si3N4±AlN±Al2O3±Y2O3 52 a Al2O3±b60±15R-YAG Al2O3±15R±15R0 ±YAG Al2O315R0 ±12H0 ±YAG Al2O3±12H0 ±21R0 ±YAG Al2O3±21R0 ±AlN±YAG 15R±15R0 ±12H±12H0 ±YAG 12H±12H0 ±21R±21R0 ±YAG 21R±21R0 ±27R±27R0 ±YAG 27R±27R0 ±2Hd ±2Hd0 ±YAG 2H±2Hd ±AlN±YAG 21R0 ±27R0 ±AlN±YAG 27R0 ±2Hd0 ±AlN±YAG 21R±27R±YAG±J0 (R) 27R±2Hd ±YAG±J0 (R) 2Hd ±AlN±YAG±J0 (R) AlN±YAG±J0 (R)±YAM AlN±YAM±J±Y2O3 b60±b25±15R±YAG b25±15R±12H±YAG b25±b10±12H±YAG b10±a0 ±12H±YAG a0 ±12H±21R±b10 a0 ±21R±b10±b8 a0 ±21R±b8±27R a0 ±b8±27R±b2 a0 ±27R±b5±2Hd a0 ±b5±2Hd ±b2 a0 ±2Hd ±b2±AlN a0 ±b2±AlN±Si3N4 a0 ±12H±21R±YAG a0 ±21R±YAG± M a0 ±21R±27R±M a0 ±27R±2Hd ±M a0 ±2H±AlN±M M±21R±YAG±J0 (R)M±21R±27R±J0 (R) M±21R±27R±J0 (R) M±27R±2Hd ±J0 (R) M±2Hd ±AlN±J0 (R) M±AlN±J0 (R)±J a YAM, 2Y2O3 .Al2O3; J, 2Y2O3 .Si2N2O; J0 (R) = 2Y2O3 .Al2O3± Y2O3 .Si2N2O; M=Si3N4 .Y2O3; 15R, 12H, 21R, 27R, 2Hd are Si±rich terminals of AlN polytypoids; 15R0 , 12H0 , 21R0 , 27R0 , 2Hd0 are Al± rich terminals of AlN polytypoids. Fig. 7. Representation of Y±Si±Al±O±N system showing phases occurring in the region bound by Si3N4, Y2O3, Al2O3, and AlN, and Si±Al±O±N behavior diagram at 1700C.52 Fig. 8. Representation of compatibility of the YAG with polytypoid phases, AlN, and Al2O3, 12 compatibility tetrahedra are formed.52 2282 V.A. Izhevskiy et al. / Journal of the European Ceramic Society 20 (2000) 2275±2295