正在加载图片...
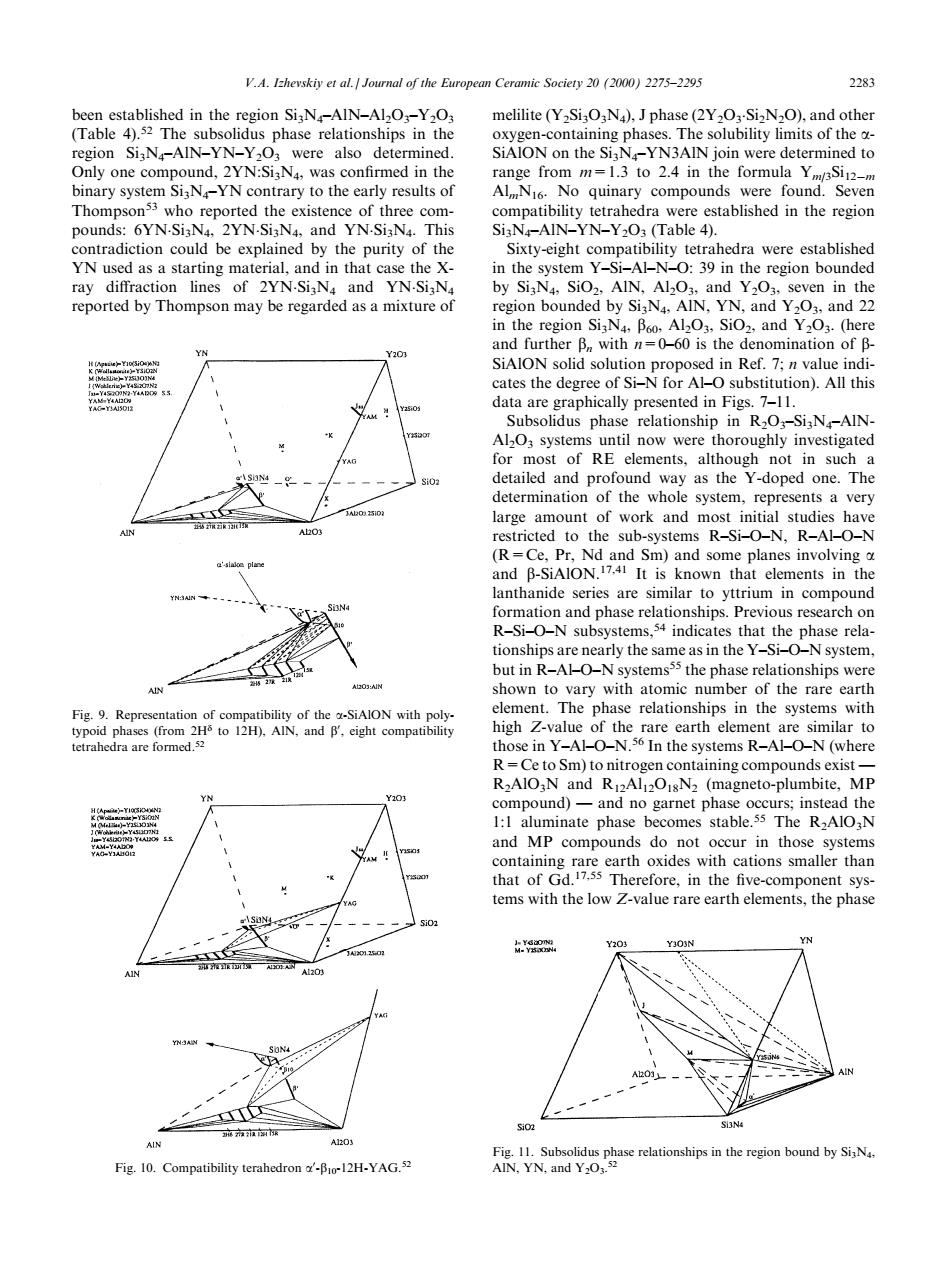
mic Society202000275-229 283 h region Si;N-AIN-YN-Y203 were also determined. regio Sixty-eight compatibility tetrahedra were established as a starting material.andnt d ch egion bounde data are graphically presented in Figs.7. now wer detailed and profound way as the Y-doped one.The determination of the whole system,represents a very hon t lanthanide series and phas tha tionships are nearly the same as in the Y-i-O-N system. but in R-AHO-N systemsss the phase relationships were to d n N(magneto-pm and MP compounds do not occur in those systems tems with the rare earth elements.the phas Fig.10.Compatibility been established in the region Si3N4±AlN±Al2O3±Y2O3 (Table 4).52 The subsolidus phase relationships in the region Si3N4±AlN±YN±Y2O3 were also determined. Only one compound, 2YN:Si3N4, was con®rmed in the binary system Si3N4±YN contrary to the early results of Thompson53 who reported the existence of three compounds: 6YN.Si3N4, 2YN.Si3N4, and YN.Si3N4. This contradiction could be explained by the purity of the YN used as a starting material, and in that case the Xray diraction lines of 2YN.Si3N4 and YN.Si3N4 reported by Thompson may be regarded as a mixture of melilite (Y2Si3O3N4), J phase (2Y2O3 .Si2N2O), and other oxygen-containing phases. The solubility limits of the aSiAlON on the Si3N4±YN3AlN join were determined to range from m=1.3 to 2.4 in the formula Ym/3Si12ÿm AlmN16. No quinary compounds were found. Seven compatibility tetrahedra were established in the region Si3N4±AlN±YN±Y2O3 (Table 4). Sixty-eight compatibility tetrahedra were established in the system Y±Si±Al±N±O: 39 in the region bounded by Si3N4, SiO2, AlN, Al2O3, and Y2O3, seven in the region bounded by Si3N4, AlN, YN, and Y2O3, and 22 in the region Si3N4, b60, Al2O3, SiO2, and Y2O3. (here and further bn with n=0±60 is the denomination of bSiAlON solid solution proposed in Ref. 7; n value indicates the degree of Si±N for Al±O substitution). All this data are graphically presented in Figs. 7±11. Subsolidus phase relationship in R2O3±Si3N4±AlNAl2O3 systems until now were thoroughly investigated for most of RE elements, although not in such a detailed and profound way as the Y-doped one. The determination of the whole system, represents a very large amount of work and most initial studies have restricted to the sub-systems R±Si±O±N, R±Al±O±N (R=Ce, Pr, Nd and Sm) and some planes involving a and b-SiAlON.17,41 It is known that elements in the lanthanide series are similar to yttrium in compound formation and phase relationships. Previous research on R±Si±O±N subsystems,54 indicates that the phase relationships are nearly the same as in the Y±Si±O±N system, but in R±Al±O±N systems55 the phase relationships were shown to vary with atomic number of the rare earth element. The phase relationships in the systems with high Z-value of the rare earth element are similar to those in Y±Al±O±N.56 In the systems R±Al±O±N (where R=Ce to Sm) to nitrogen containing compounds exist Ð R2AlO3N and R12Al12O18N2 (magneto-plumbite, MP compound) Ð and no garnet phase occurs; instead the 1:1 aluminate phase becomes stable.55 The R2AlO3N and MP compounds do not occur in those systems containing rare earth oxides with cations smaller than that of Gd.17,55 Therefore, in the ®ve-component systems with the low Z-value rare earth elements, the phase Fig. 9. Representation of compatibility of the a-SiAlON with polytypoid phases (from 2Hd to 12H), AlN, and b0 , eight compatibility tetrahedra are formed.52 Fig. 10. Compatibility terahedron a0 -b10-12H-YAG.52 Fig. 11. Subsolidus phase relationships in the region bound by Si3N4, AlN, YN, and Y2O3. 52 V.A. Izhevskiy et al. / Journal of the European Ceramic Society 20 (2000) 2275±2295 2283