正在加载图片...
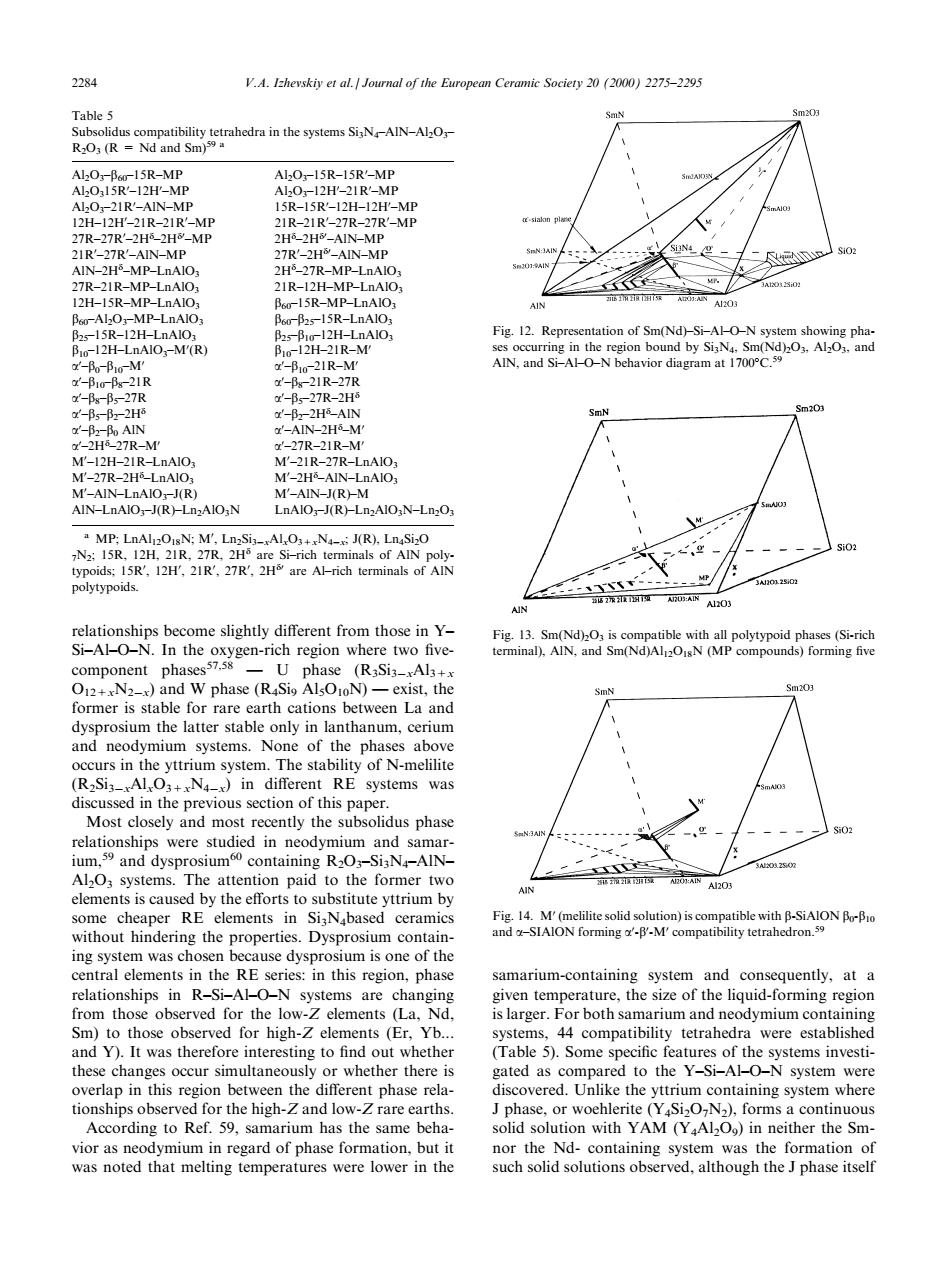
2284 V.A.I-hevskiy et al Journal of the European Ceramic Society 2(2)2275-2295 (odra in the N AIN-AO 15R-MP AlOr-15R-15R'-MP MP H-RN 27- HE-MP MP-LnAIO 27R-MB-I 12H-I5R-MP-LnAIC 5R-MP-IDAIC Fig.12.Represe IN.and Si-AH avior diagram at1700° M-77R-7H AIO.N LNO:R).N-LnO MP::M'.LnSi-AL.-J(R).LnaSi:O phase (RaSia-Als O+N2)and W phase (R Si AlsOoN)-exist,the former is stable for rare earth cations between La and occurs in the yttrium system.The stability of N-melilite (R2Si3-AlO3+N in different RE systems was Most nt the phase relationships were studied in neodymium and samar ium,and dysprosium containing R2O-SiaN-AIN- AlOs system tion paid to the former two ihB-SiAION without hindering the properties.Dysprosium contain- in R-Si-ALO-N rium-containing system from those observed for the(La. islarger.For both samarium and neodymium containing Sm)to those observed for high-Z elements (Er.Yb.. and Y) to nn overlan in this region hetween the different phase rela discovered.Unlike the yttrium containing system where 59, Jphase.or wochlerite (),forms a continuous arium has the ame ber solid solution with AM(YAl2O)in neither the Smrelationships become slightly dierent from those in Y± Si±Al±O±N. In the oxygen-rich region where two ®vecomponent phases57,58 Ð U phase (R3Si3ÿxAl3+x O12+xN2ÿx) and W phase (R4Si9 Al5O10N) Ð exist, the former is stable for rare earth cations between La and dysprosium the latter stable only in lanthanum, cerium and neodymium systems. None of the phases above occurs in the yttrium system. The stability of N-melilite (R2Si3ÿxAlxO3+xN4ÿx) in dierent RE systems was discussed in the previous section of this paper. Most closely and most recently the subsolidus phase relationships were studied in neodymium and samarium,59 and dysprosium60 containing R2O3±Si3N4±AlN± Al2O3 systems. The attention paid to the former two elements is caused by the eorts to substitute yttrium by some cheaper RE elements in Si3N4based ceramics without hindering the properties. Dysprosium containing system was chosen because dysprosium is one of the central elements in the RE series: in this region, phase relationships in R±Si±Al±O±N systems are changing from those observed for the low-Z elements (La, Nd, Sm) to those observed for high-Z elements (Er, Yb... and Y). It was therefore interesting to ®nd out whether these changes occur simultaneously or whether there is overlap in this region between the dierent phase relationships observed for the high-Z and low-Z rare earths. According to Ref. 59, samarium has the same behavior as neodymium in regard of phase formation, but it was noted that melting temperatures were lower in the samarium-containing system and consequently, at a given temperature, the size of the liquid-forming region is larger. For both samarium and neodymium containing systems, 44 compatibility tetrahedra were established (Table 5). Some speci®c features of the systems investigated as compared to the Y±Si±Al±O±N system were discovered. Unlike the yttrium containing system where J phase, or woehlerite (Y4Si2O7N2), forms a continuous solid solution with YAM (Y4Al2O9) in neither the Smnor the Nd- containing system was the formation of such solid solutions observed, although the J phase itself Table 5 Subsolidus compatibility tetrahedra in the systems Si3N4±AlN±Al2O3± R2O3 (R = Nd and Sm)59 a Al2O3±b60±15R±MP Al2O3±15R±15R0 ±MP Al2O315R0 ±12H0 ±MP Al2O3±12H0 ±21R0 ±MP Al2O3±21R0 ±AlN±MP 15R±15R0 ±12H±12H0 ±MP 12H±12H0 ±21R±21R0 ±MP 21R±21R0 ±27R±27R0 ±MP 27R±27R0 ±2Hd ±2Hd0 ±MP 2Hd ±2Hd0 ±AlN±MP 21R0 ±27R0 ±AlN±MP 27R0 ±2Hd0 ±AlN±MP AlN±2Hd ±MP±LnAlO3 2Hd ±27R±MP±LnAlO3 27R±21R±MP±LnAlO3 21R±12H±MP±LnAlO3 12H±15R±MP±LnAlO3 b60±15R±MP±LnAlO3 b60±Al2O3±MP±LnAlO3 b60±b25±15R±LnAlO3 b25±15R±12H±LnAlO3 b25±b10±12H±LnAlO3 b10±12H±LnAlO3±M0 (R) b10±12H±21R±M0 a0 ±b0±b10±M0 a0 ±b10±21R±M0 a0 ±b10±b8±21R a0 ±b8±21R±27R a0 ±b8±b5±27R a0 ±b5±27R±2Hd a0 ±b5±b2±2Hd a0 ±b2±2Hd ±AlN a0 ±b2±b0 AlN a0 ±AlN±2Hd ±M0 a0 ±2Hd ±27R±M0 a0 ±27R±21R±M0 M0 ±12H±21R±LnAlO3 M0 ±21R±27R±LnAlO3 M0 ±27R±2Hd ±LnAlO3 M0 ±2Hd ±AlN±LnAlO3 M0 ±AlN±LnAlO3±J(R) M0 ±AlN±J(R)±M AlN±LnAlO3±J(R)±Ln2AlO3N LnAlO3±J(R)±Ln2AlO3N±Ln2O3 a MP; LnAl12O18N; M0 , Ln2Si3ÿxAlxO3+xN4ÿx; J(R), Ln4Si2O 7N2; 15R, 12H, 21R, 27R, 2Hd are Si±rich terminals of AlN polytypoids; 15R0 , 12H0 , 21R0 , 27R0 , 2Hd0 are Al±rich terminals of AlN polytypoids. Fig. 12. Representation of Sm(Nd)±Si±Al±O±N system showing phases occurring in the region bound by Si3N4, Sm(Nd)2O3, Al2O3, and AlN, and Si±Al±O±N behavior diagram at 1700C.59 Fig. 13. Sm(Nd)2O3 is compatible with all polytypoid phases (Si-rich terminal), AlN, and Sm(Nd)Al12O18N (MP compounds) forming ®ve Fig. 14. M0 (melilite solid solution) is compatible with b-SiAlON b0-b10 and a±SIAlON forming a0 -b0 -M0 compatibility tetrahedron.59 2284 V.A. Izhevskiy et al. / Journal of the European Ceramic Society 20 (2000) 2275±2295