正在加载图片...
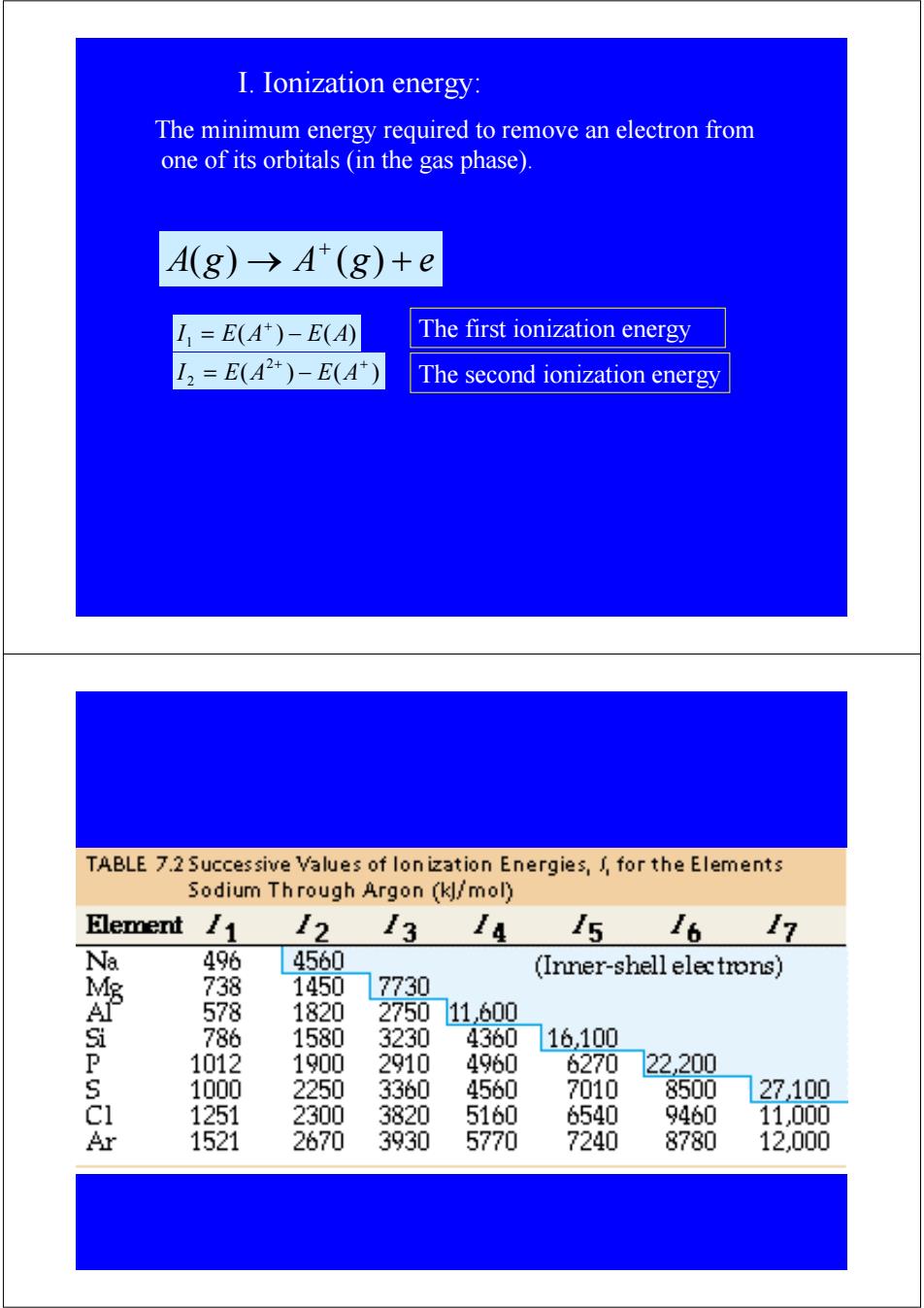
I.Ionization energy: The minimum energy required to remove an electron from one of its orbitals (in the gas phase) A(8)→A(8) +e =E(A)-E(A) The first ionization energy I2=E(A2+)-E(A) The second ionization energy TABLE 7.2 Successive Values of lon ization Energies,for the Elements Sodium Through Argon (kl/mol) Element 11 12 3 14 5 /6 /7 Na 496 4560 (Inner-shell electrons) 738 1450 17730 A 578 1820 275011600 786 1580 3230 4360 16100 P 1012 1900 2910 4960 6270 22.200 1000 2250 3360 4560 7010 8500 27100 1251 2300 3820 5160 6540 9460 11,000 Ar 1521 2670 3930 5770 7240 8780 12,000The minimum energy required to remove an electron from one of its orbitals (in the gas phase). A g → A g + e + ( ) ( ) ( ) ( ) I1 = E A − E A + ( ) ( ) 2 2 + + I = E A − E A The first ionization energy The second ionization energy I. Ionization energy: