正在加载图片...
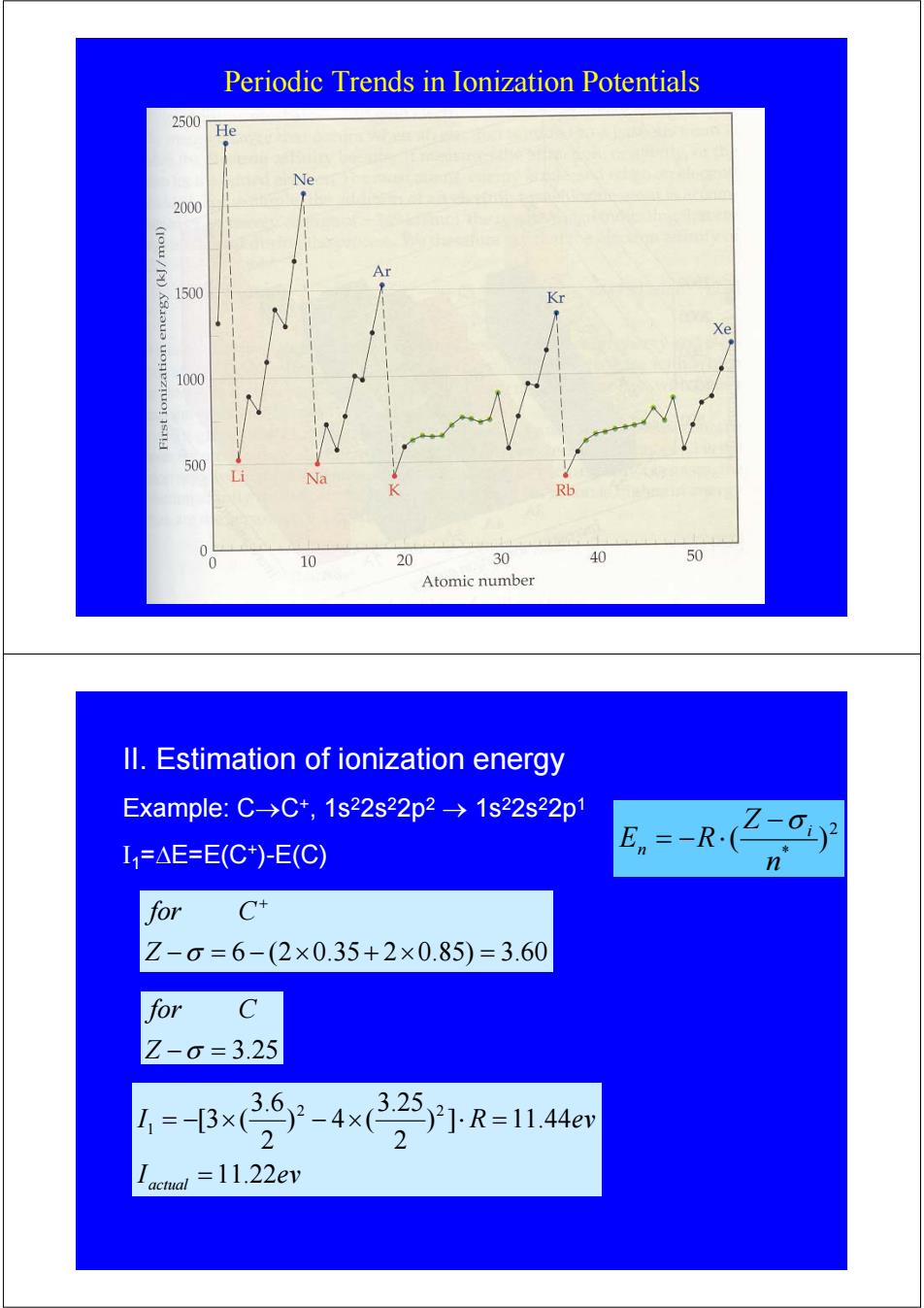
Periodic Trends in Ionization Potentials 2500 He Ne 2000 Ar 1500 Kr Xe 1000 500 Na Rb 10 20 30 40 50 Atomic number ll.Estimation of ionization energy Example:C-→C*,1s22s22p2→1s22s22p1 I1=△E=E(C*)-E(C) E.--R-(Z-Gy n for C+ Z-0=6-(2×0.35+2×0.85)=3.60 for C Z-6=3.25 =B✉9-4R=44 actal =11.22evPeriodic Trends in Ionization Potentials II. Estimation of ionization energy Example: C→C+, 1s22s22p2 → 1s22s22p1 I1=ΔE=E(C+)-E(C) − = 6 − (2×0.35 + 2×0.85) = 3.60 + Z σ for C 2 * ( ) n Z E R i n −σ = − ⋅ Z −σ = 3.25 for C I ev I R ev actual 11.22 ) ] 11.44 2 3.25 ) 4 ( 2 3.6 [3 ( 2 2 1 = = − × − × ⋅ =