正在加载图片...
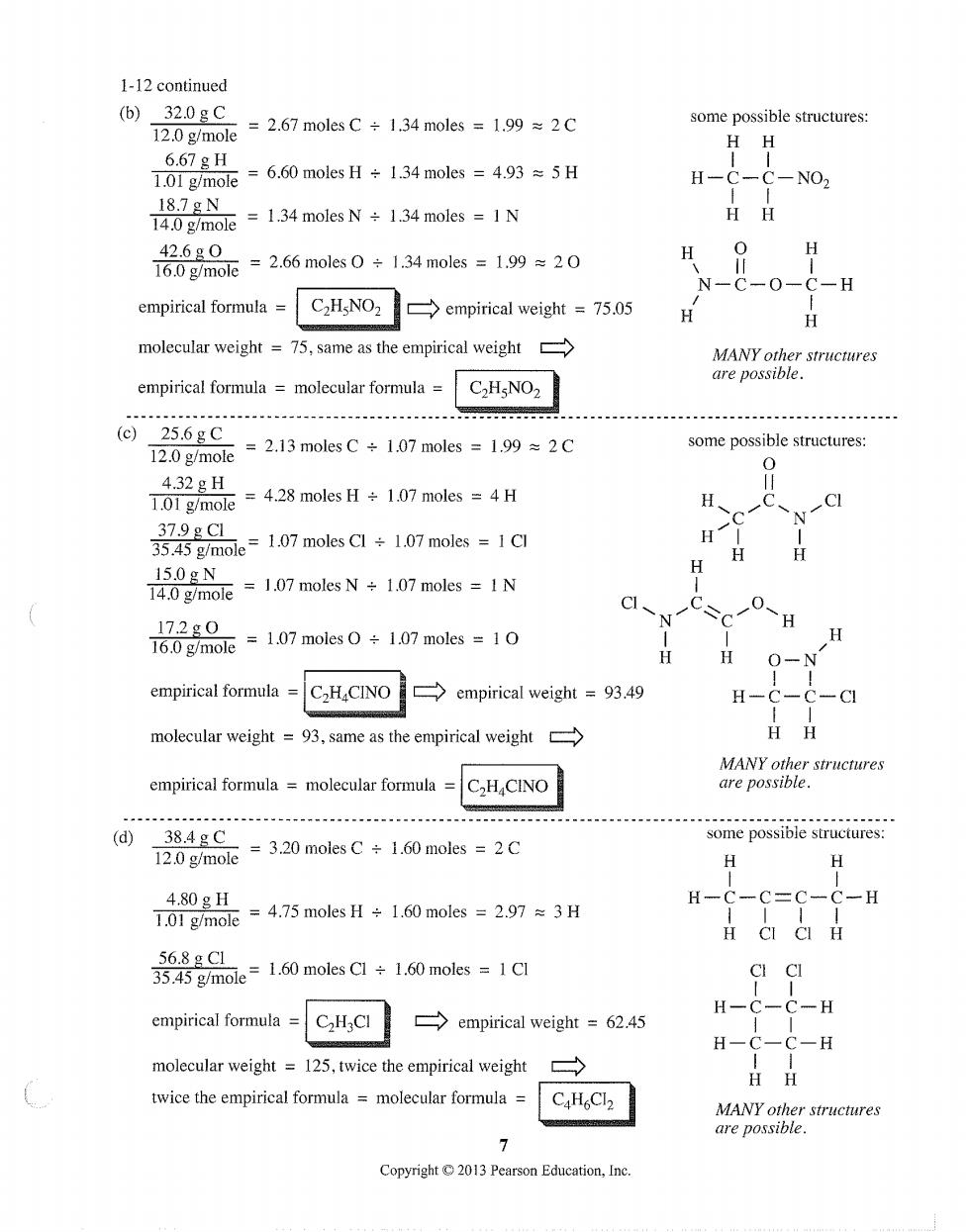
1-12 continued (b)32.0gC =2.67 moles C÷1.34 moles=1.99≈2C some possible structures: 12.0 g/mole H-C-C-NO2 m1moleN1 mlIN HH 760gm0=2.66 moles0÷134 moles=199=20 42.6g0 H N-C-0-C-H empirical formula C2HgNO2 empirical weight =75.05 H molecular weight=75,same as the empirical weight MANY other structures are possible. empirical formula molecular formula C.HsNO2 (c)25.6gC T2.0g/mole =2.13 moles C1.07 moles=1992C some possible structures: 4.32gH 101gm nole 4.28 moles H+1.07 moles =4 H CI 35.45 g/mole=1.07 moles Cl+1.07 moles =1Cl 37.9gC1 H H 140mole =1.07 moles N+1.07 moles =IN 15.0gN 16.0g/mole =1.07 moles1.07 moles =1 17.2g0 H H O-N empirical formula =C2HCINO empirical weight =93.49 H-C -CI molecular weight=93.same as the empirical weight HH MANY other structures empirical formula =molecular formula =CHCINO are possible. (d)38.4gC some possible structures: 12.0 g/mole =3.20 moles C 1.60 moles 2C H H 4.80gH 70gmoe=4.75 moles H÷1.60 moles=2.97≈3H 3g品e=160 moleC÷160nols=1d CI CI C-H empirical formula H C2H3CI empirical weight =62.45 molecular weight=125,twice the empirical weight twice the empirical formula molecular formula CaHCl2 MANY other structures are possible. 7 Copyright2013 Pearson Education,Ine