正在加载图片...
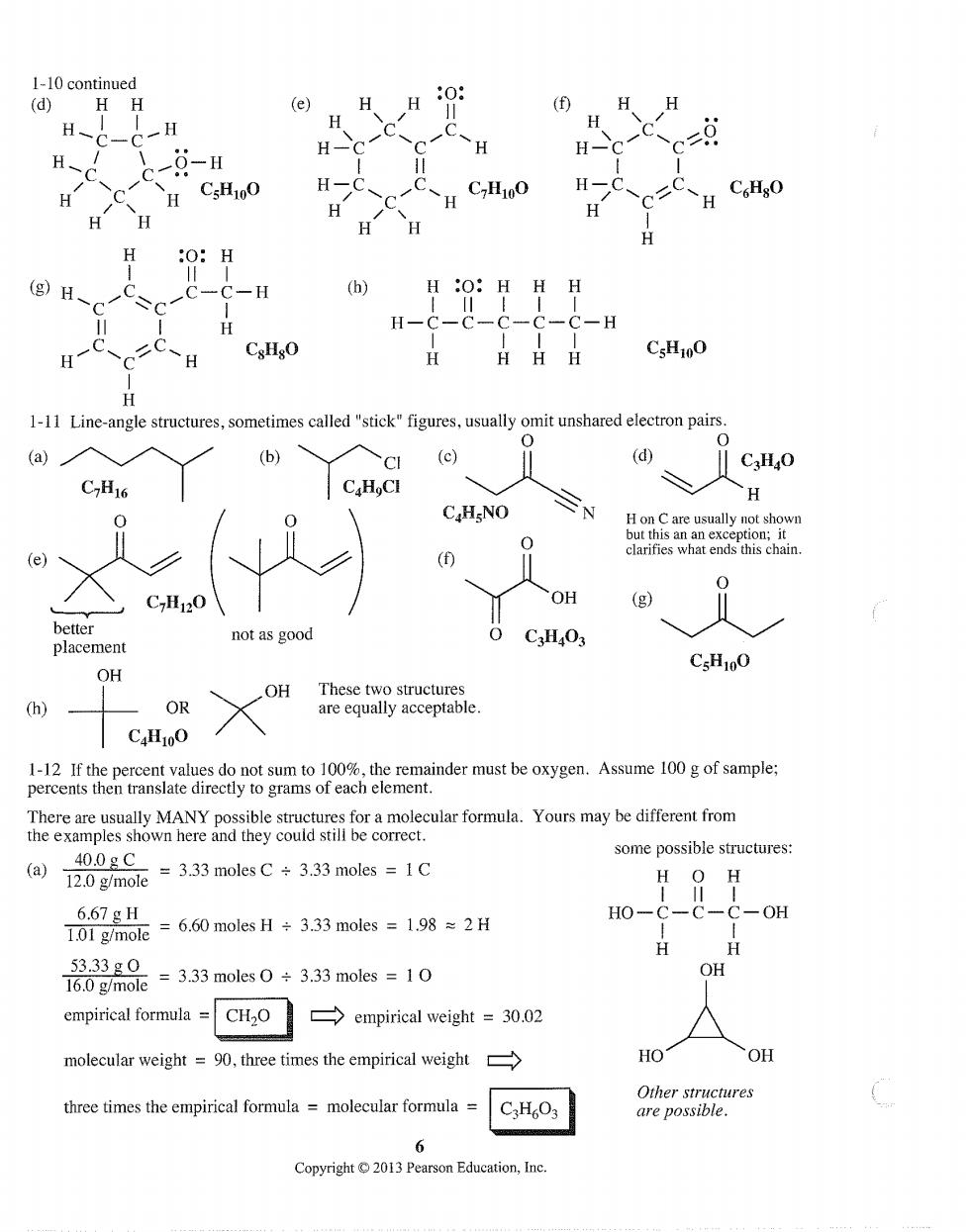
1-10 continued (d) HH (e) H H H- 0-H g)H. C.HO H日H CsH1oO 1-11 Line-angle structures,sometimes called"stick"figures,usually omit unshared electron pairs. (b) cI (c) ‖CgHO C7H16 H H on C are ends this chair C7H120 OH (g) tter not as good C3HO3 placement OH CsH100 OR CaH10O remainder must be oxygen.Assume 100g of sample; ams of each element. There are usually MANY possible structures for a molecular formula.Yours may be different from the examples shown here and they could still be correct. 40.0gC some possible structures: (a)2.0 g/mole 3.33 moles C 3.33 moles 1C H 6.67gH 101gym0c=6.60 molesH÷3.33 moles=1.98≈2H HO-C-C-C-OH 16.0 g/mole =3.33 molesO+3.3 moles =10 53.33g0 OH empirical formula=CHO empirical weight 30.02 molecular weight =9,three times the empirical weight HO OH three times the empirical formula=molecular formula Other structures C3H6O3 are possible. Pearson Education,Ine