正在加载图片...
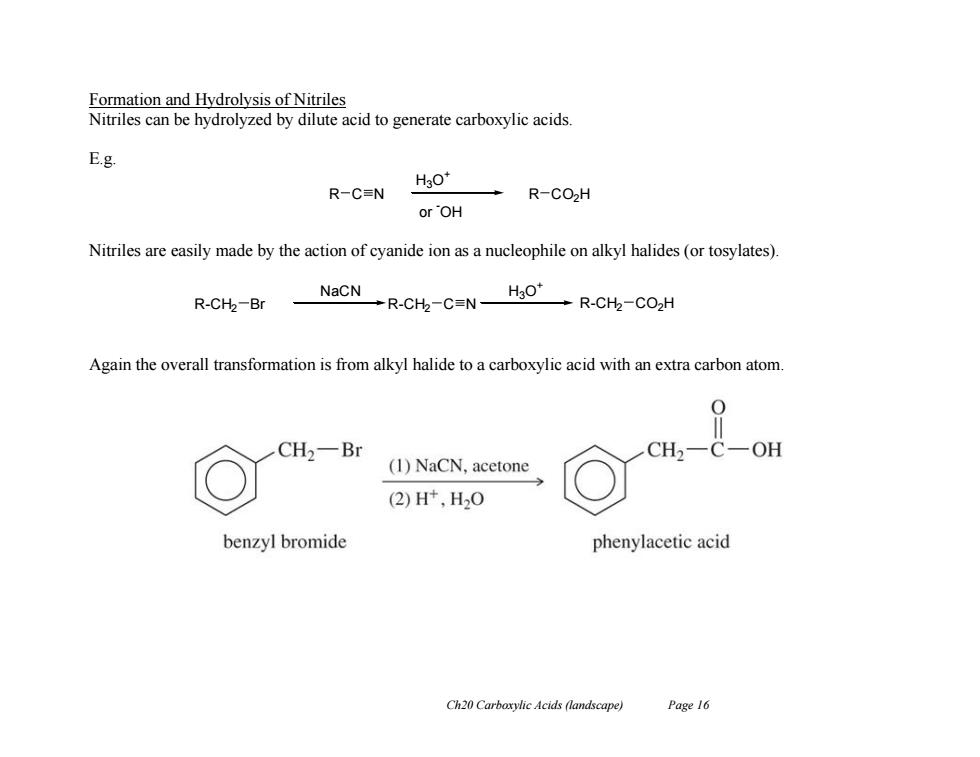
Formation and Hydrolysis of Nitriles Nitriles can be hydrolyzed by dilute acid to generate carboxylic acids E.g. H3O* R一C≡N R-CO2H or OH Nitriles are easily made by the action of cyanide ion as a nucleophile on alkyl halides(or tosylates). NaCN R-CH2-Br →R-Ch-CNHsO →R-CH-CO2H Again the overall transformation is from alkyl halide to a carboxylic acid with an extra carbon atom. CH2一Br CH -OH (1)NaCN,acetone (2)Ht,H20 benzyl bromide phenylacetic acid Ch20 Carboxylic Acids (landscape) Page16 Ch20 Carboxylic Acids (landscape) Page 16 Formation and Hydrolysis of Nitriles Nitriles can be hydrolyzed by dilute acid to generate carboxylic acids. E.g. Nitriles are easily made by the action of cyanide ion as a nucleophile on alkyl halides (or tosylates). Again the overall transformation is from alkyl halide to a carboxylic acid with an extra carbon atom. R C N H3O + or -OH R CO2H R-CH2 C N H3O + R-CH2 Br R-CH2 CO2H NaCN