正在加载图片...
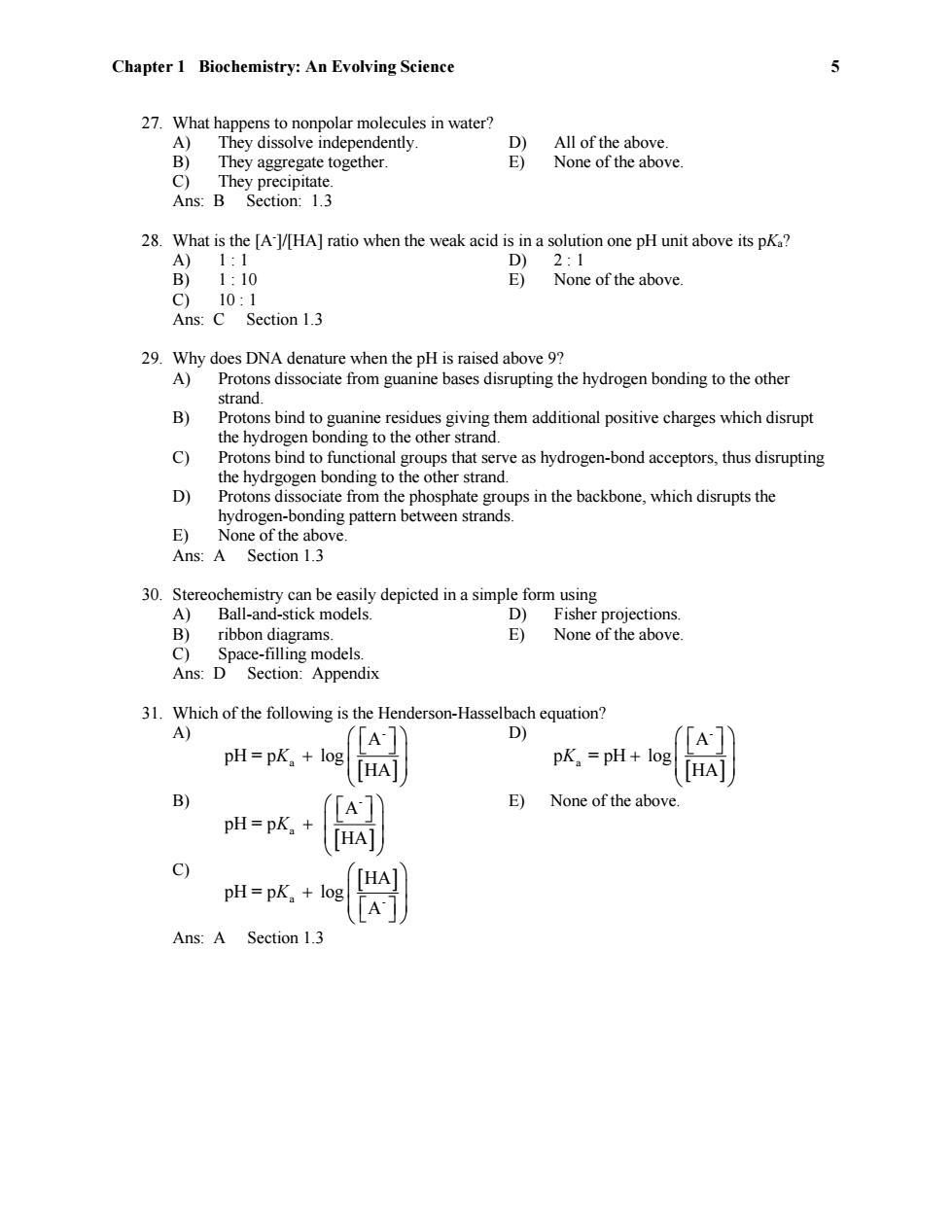
Chapter 1 Biochemistry:An Evolving Science 5 27.What happens to nonpolar molecules in water? A) They dissolve independently. D) All of the above. B) They aggregate together. E) None of the above. C)They precipitate. Ans:B Section:1.3 28.What is the [A-l/HA]ratio when the weak acid is in a solution one pH unit above its pKa? A)1:1 D) 2:1 B)1:10 E) None of the above. C)10:1 Ans:C Section 1.3 29.Why does DNA denature when the pH is raised above 9? A)Protons dissociate from guanine bases disrupting the hydrogen bonding to the other strand. B) Protons bind to guanine residues giving them additional positive charges which disrupt the hydrogen bonding to the other strand. c) Protons bind to functional groups that serve as hydrogen-bond acceptors,thus disrupting the hydrgogen bonding to the other strand. D) Protons dissociate from the phosphate groups in the backbone,which disrupts the hydrogen-bonding pattern between strands. E)None of the above. Ans:A Section 1.3 30.Stereochemistry can be easily depicted in a simple form using A) Ball-and-stick models. D)Fisher projections. B)ribbon diagrams. E)None of the above. C) Space-filling models. Ans:D Section:Appendix 31.Which of the following is the Henderson-Hasselbach equation? A) D) A pH=pK log pK。=pH+log HA HA B) E) None of the above. pH=pK. pH=pK。+ Ans:A Section 1.3Chapter 1 Biochemistry: An Evolving Science 5 27. What happens to nonpolar molecules in water? A) They dissolve independently. D) All of the above. B) They aggregate together. E) None of the above. C) They precipitate. Ans: B Section: 1.3 28. What is the [A-]/[HA] ratio when the weak acid is in a solution one pH unit above its pKa? A) 1 : 1 D) 2 : 1 B) 1 : 10 E) None of the above. C) 10 : 1 Ans: C Section 1.3 29. Why does DNA denature when the pH is raised above 9? A) Protons dissociate from guanine bases disrupting the hydrogen bonding to the other strand. B) Protons bind to guanine residues giving them additional positive charges which disrupt the hydrogen bonding to the other strand. C) Protons bind to functional groups that serve as hydrogen-bond acceptors, thus disrupting the hydrgogen bonding to the other strand. D) Protons dissociate from the phosphate groups in the backbone, which disrupts the hydrogen-bonding pattern between strands. E) None of the above. Ans: A Section 1.3 30. Stereochemistry can be easily depicted in a simple form using A) Ball-and-stick models. D) Fisher projections. B) ribbon diagrams. E) None of the above. C) Space-filling models. Ans: D Section: Appendix 31. Which of the following is the Henderson-Hasselbach equation? A) - a A pH = p log HA K D) - a A p = pH log HA K B) - a A pH = p HA K E) None of the above. C) a - HA pH = p log A K Ans: A Section 1.3