正在加载图片...
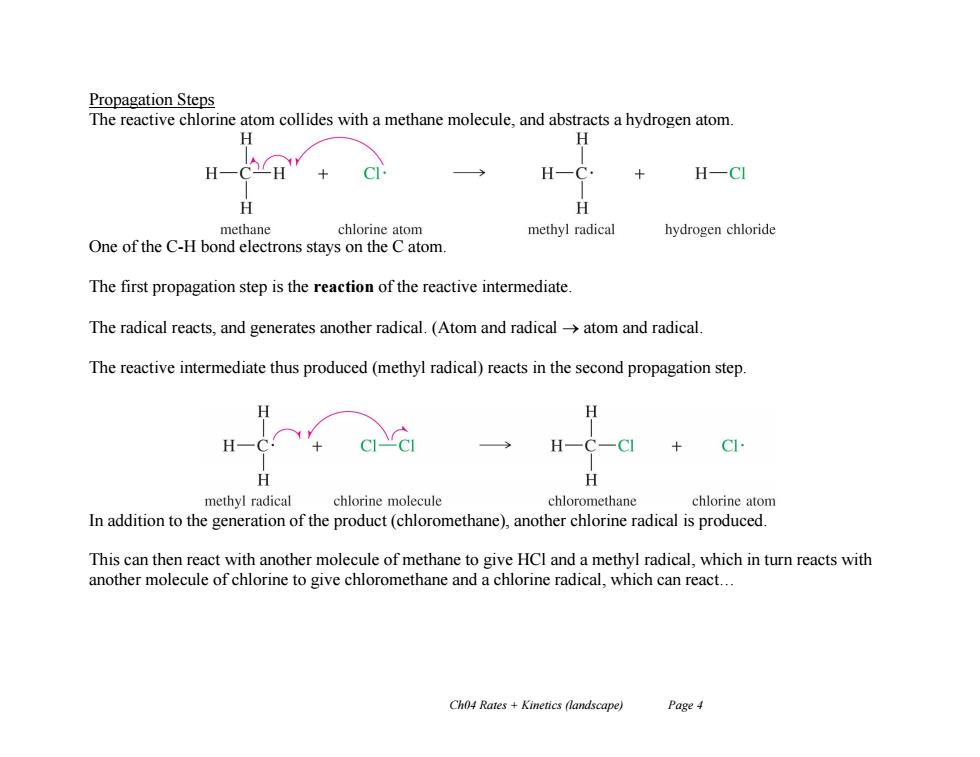
Propagation Steps The reactive chlorine atom collides with a methane molecule,and abstracts a hydrogen atom H H H-CH C H一 H-CI H H methane chlorine atom methyl radical hydrogen chloride One of the C-H bond electrons stays on the C atom. The first propagation step is the reaction of the reactive intermediate. The radical reacts,and generates another radical.(Atom and radical->atom and radical The reactive intermediate thus produced(methyl radical)reacts in the second propagation step. H C H methyl radical chlorine molecule chloromethane chlorine atom In addition to the generation of the product(chloromethane),another chlorine radical is produced. This can then react with another molecule of methane to give HCl and a methyl radical,which in turn reacts with another molecule of chlorine to give chloromethane and a chlorine radical,which can react... Ch04 Rates +Kinetics (landscape) Page 4 Ch04 Rates + Kinetics (landscape) Page 4 Propagation Steps The reactive chlorine atom collides with a methane molecule, and abstracts a hydrogen atom. One of the C-H bond electrons stays on the C atom. The first propagation step is the reaction of the reactive intermediate. The radical reacts, and generates another radical. (Atom and radical atom and radical. The reactive intermediate thus produced (methyl radical) reacts in the second propagation step. In addition to the generation of the product (chloromethane), another chlorine radical is produced. This can then react with another molecule of methane to give HCl and a methyl radical, which in turn reacts with another molecule of chlorine to give chloromethane and a chlorine radical, which can react…