正在加载图片...
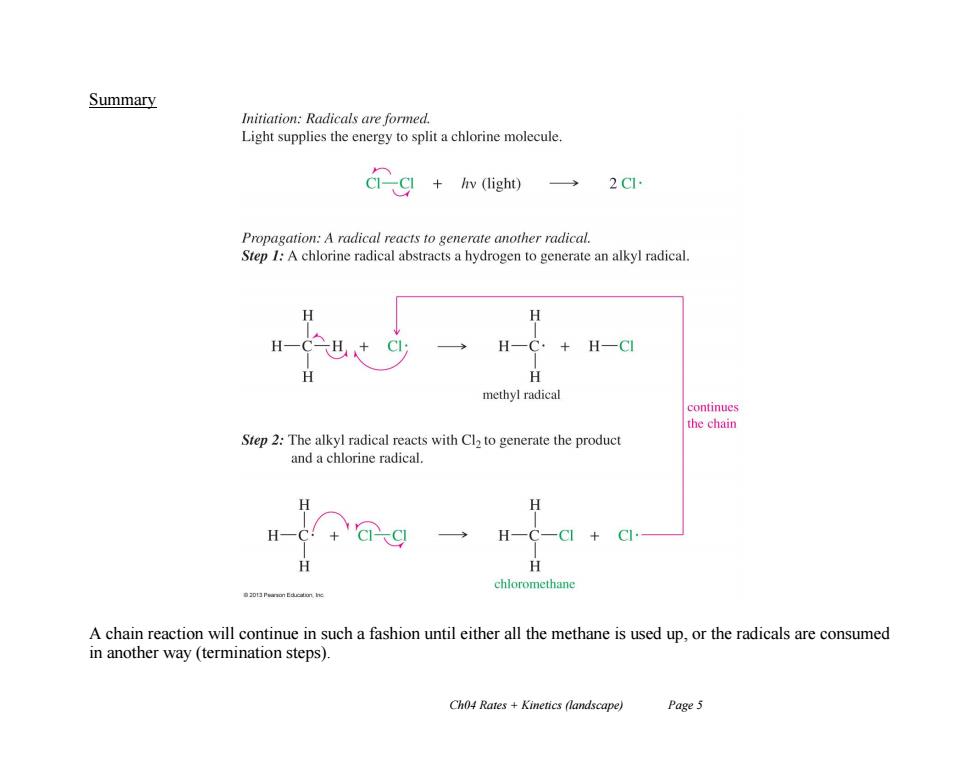
Summary Initiation:Radicals are formed. Light supplies the energy to split a chlorine molecule. Gg+wigh一 Propagation:A radical reacts to generate another radical. Step I:A chlorine radical abstracts a hydrogen to generate an alkyl radical. H-C. +HCI H methyl radical continues the chain Step 2:The alkyl radical reacts with Cl2 to generate the product and a chlorine radical. H H H chloromethane 20Pearmn Eaucatunt he A chain reaction will continue in such a fashion until either all the methane is used up,or the radicals are consumed in another way (termination steps). Ch04 Rates +Kinetics (landscape) Page 5Ch04 Rates + Kinetics (landscape) Page 5 Summary A chain reaction will continue in such a fashion until either all the methane is used up, or the radicals are consumed in another way (termination steps)