正在加载图片...
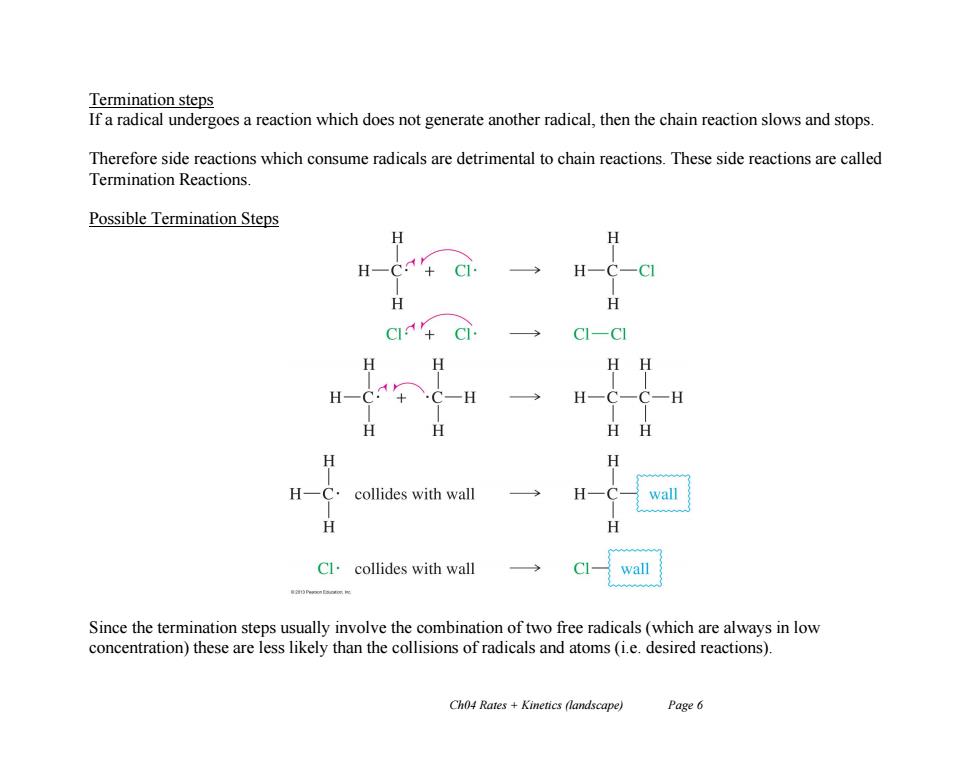
Termination steps If a radical undergoes a reaction which does not generate another radical,then the chain reaction slows and stops. Therefore side reactions which consume radicals are detrimental to chain reactions.These side reactions are called Termination Reactions. Possible Termination Steps H H-C H H -C] H HH H collides with wall wall Cl.collides with wall CI- wall Since the termination steps usually involve the combination of two free radicals(which are always in low concentration)these are less likely than the collisions of radicals and atoms (i.e.desired reactions). Ch04 Rates Kinetics (landscape) Page 6 Ch04 Rates + Kinetics (landscape) Page 6 Termination steps If a radical undergoes a reaction which does not generate another radical, then the chain reaction slows and stops. Therefore side reactions which consume radicals are detrimental to chain reactions. These side reactions are called Termination Reactions. Possible Termination Steps Since the termination steps usually involve the combination of two free radicals (which are always in low concentration) these are less likely than the collisions of radicals and atoms (i.e. desired reactions)