正在加载图片...
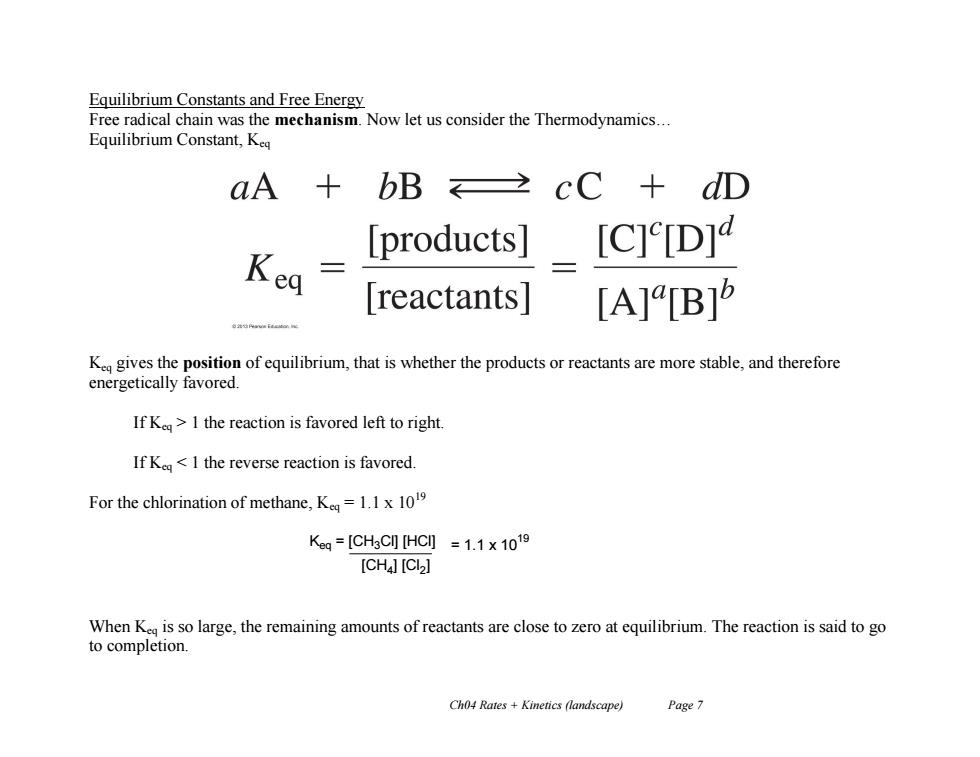
Equilibrium Constants and Free Energy Free radical chain was the mechanism.Now let us consider the Thermodynamics... Equilibrium Constant,Keq aA bB cC+dD products] [C]e[D] Kea reactants| [A][B]5 K gives the position of equilibrium,that is whether the products or reactants are more stable,and therefore energetically favored. If Ke>I the reaction is favored left to right. If Kea<1 the reverse reaction is favored. For the chlorination of methane,K=1.1x10 Kg=[CH3CHC]=1.1×1019 [CH4][CI>] When K is so large,the remaining amounts of reactants are close to zero at equilibrium.The reaction is said to go to completion. Ch04 Rates Kinetics (landscape) Page 7Ch04 Rates + Kinetics (landscape) Page 7 Equilibrium Constants and Free Energy Free radical chain was the mechanism. Now let us consider the Thermodynamics… Equilibrium Constant, Keq Keq gives the position of equilibrium, that is whether the products or reactants are more stable, and therefore energetically favored. If Keq > 1 the reaction is favored left to right. If Keq < 1 the reverse reaction is favored. For the chlorination of methane, Keq = 1.1 x 1019 When Keq is so large, the remaining amounts of reactants are close to zero at equilibrium. The reaction is said to go to completion. Keq = [CH3Cl] [HCl] [CH4 ] [Cl2 ] = 1.1 x 10 19