正在加载图片...
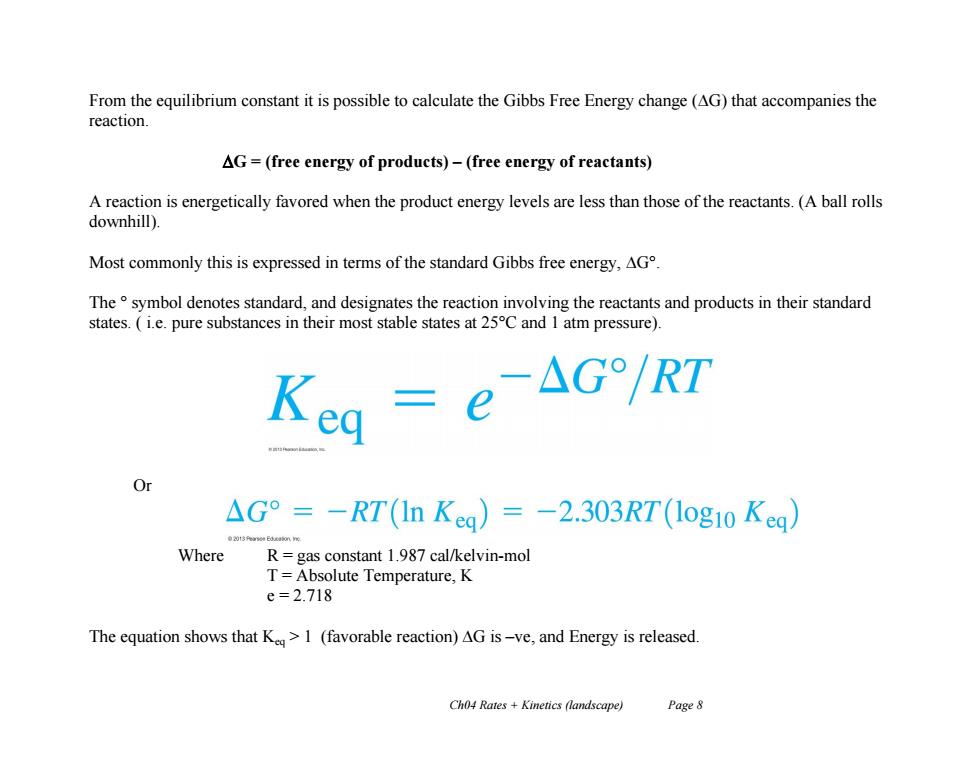
From the equilibrium constant it is possible to calculate the Gibbs Free Energy change (AG)that accompanies the reaction. AG=(free energy of products)-(free energy of reactants) A reaction is energetically favored when the product energy levels are less than those of the reactants.(A ball rolls downhill). Most commonly this is expressed in terms of the standard Gibbs free energy,AG The symbol denotes standard,and designates the reaction involving the reactants and products in their standard states.(ie.pure substances in their most stable states at 25C and 1 atm pressure). △G°=-RT(In Keg)=-2.303RT(1og10Kea) Where R=gas constant 1.987 cal/kelvin-mol T=Absolute Temperature,K e=2.718 The equation shows that K>1 (favorable reaction)AG is-ve,and Energy is released. Ch04 Rates Kinetics (landscape) Page 8 Ch04 Rates + Kinetics (landscape) Page 8 From the equilibrium constant it is possible to calculate the Gibbs Free Energy change (G) that accompanies the reaction. G = (free energy of products) – (free energy of reactants) A reaction is energetically favored when the product energy levels are less than those of the reactants. (A ball rolls downhill). Most commonly this is expressed in terms of the standard Gibbs free energy, G°. The ° symbol denotes standard, and designates the reaction involving the reactants and products in their standard states. ( i.e. pure substances in their most stable states at 25°C and 1 atm pressure). Or Where R = gas constant 1.987 cal/kelvin-mol T = Absolute Temperature, K e = 2.718 The equation shows that Keq > 1 (favorable reaction) G is –ve, and Energy is released