正在加载图片...
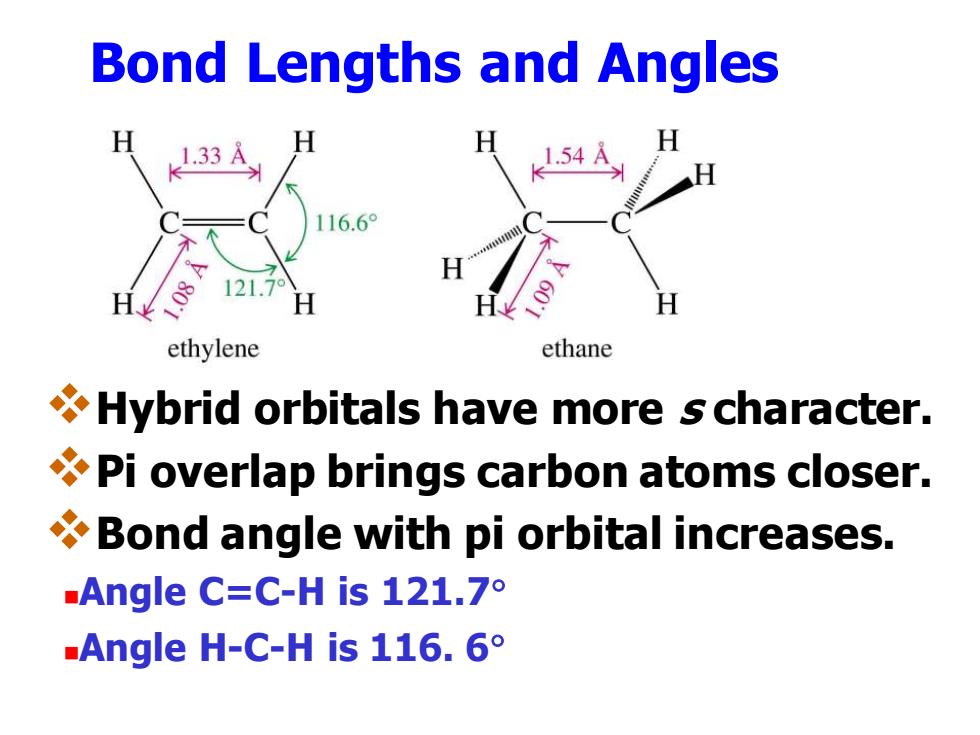
Bond Lengths and Angles 1.33 54 116.6° 121.7 ethylene ethane Hybrid orbitals have more s character. Pi overlap brings carbon atoms closer. Bond angle with pi orbital increases. Angle C:=C-His121.7° Angle H-C-His116.6° Bond Lengths and Angles ❖Hybrid orbitals have more s character. ❖Pi overlap brings carbon atoms closer. ❖Bond angle with pi orbital increases. ◼Angle C=C-H is 121.7 ◼Angle H-C-H is 116. 6